Chapter 5: Molecules, Compounds, and Chemical Equations
Chapter 5 Practice
5.1 Ionic Bonding [Go to section 5.1]
- Explain why a sample of copper hydroxide, composed of [latex]\ce{Cu^{2+}}[/latex] and [latex]\ce{OH}^-[/latex] ions, is neutral.
- Which of the following atoms would be expected to form negative ions in binary ionic compounds and which would be expected to form positive ions: [latex]\ce{Br, Ca, Na, N, F, Al, Sn, S, Cd}[/latex]?
- Predict the charge on the following ions if they are found in binary ionic compounds: [latex]\ce{Br, Ca, Li, S}[/latex].
Show Selected Solutions
- In order to maintain charge neutrality, the substance contains a ratio of [latex]\ce{Cu}^{2+}[/latex] to [latex]\ce{OH^-}[/latex] of 1:2.
- The answers are as follows
- [latex]\ce{Br}: \ce{Br^-}[/latex]
- [latex]\ce{Ca}: \ce{Ca^{2+}}[/latex]
- [latex]\ce{Li}: \ce{Li^+}[/latex]
- [latex]\ce{S}: \ce{S^{2-}}[/latex]
5.3 Molecular and Ionic Compounds [Go to section 5.3]
- For each of the following pairs of ions, write the symbol for the formula of the compound they will form:
- [latex]\ce{K^+, O^{2-}}[/latex]
- [latex]\ce{NH^+_4, PO^{3-}_4}[/latex]
- [latex]\ce{Al^{3+}, O^{2-}}[/latex]
- [latex]\ce{Na^_+, CO^{2-}_3}[/latex]
- [latex]\ce{Ba^{2+}, PO^{3-}_4}[/latex]
- Using the periodic table, predict whether the following chlorides are ionic or covalent: [latex]\ce{KCl, NCl3, ICl, MgCl2, PCl5}, \text{ and } \ce{CCl4}[/latex].
- For each of the following compounds, state whether it is ionic or covalent. If it is ionic, write the symbols for the ions involved:
- [latex]\ce{NF3}[/latex]
- [latex]\ce{BaO }[/latex]
- [latex]\ce{(NH4)2CO3 }[/latex]
- [latex]\ce{Sr(H2PO4)2 }[/latex]
- [latex]\ce{IBr }[/latex]
- [latex]\ce{Na2O }[/latex]
Show Selected Solutions
- In general, those elements that are widely separated in the periodic table—that is, at the extreme left and extreme right—will form compounds that are ionic. Those elements that are near one another in the periodic table generally will form covalent compounds. More specifically, when a metal is combined with one or more nonmetals, the compound is usually ionic. Covalent compounds are usually formed by a combination of nonmetals. Ionic: [latex]\ce{KCl, MgCl2}[/latex]; Covalent: [latex]\ce{NCl3, ICl, PCl5, CCl4}[/latex]
5.4 Chemical Nomenclature [Go to section 5.4]
- Name the following compounds:
- [latex]\ce{BeCl2}[/latex]
- [latex]\ce{HBr}[/latex]
- [latex]\ce{AlF3}[/latex]
- Name the following compounds:
- [latex]\ce{NaF}[/latex]
- [latex]\ce{Rb2O}[/latex]
- [latex]\ce{BCl3}[/latex]
- [latex]\ce{H2Se}[/latex]
- [latex]\ce{P4O6}[/latex]
- [latex]\ce{ICl3}[/latex]
- Write the formulas of the following compounds:
- calcium chloride
- hydrogen fluoride
- gallium phosphide
- aluminum bromide
- ammonium sulfate
- Write the formulas of the following compounds:
- lithium carbonate
- sodium perchlorate
- barium hydroxide
- ammonium carbonate
- sulfuric acid
- calcium acetate
- magnesium phosphate
- sodium sulfite
- Write the formulas of the following compounds:
- silver(I) sulfide
- aluminum fluoride trihydrate
- silicon dioxide
- Write the formulas of the following compounds:
- barium chloride
- magnesium nitride
- sulfur dioxide
- nitrogentrichloride
- dinitrogen trioxide
- tin(IV) chloride
- Each of the following compounds contains a metal that can exhibit more than one ionic charge. Name these compounds:
- [latex]\ce{TiCl4}[/latex]
- [latex]\ce{CoCl2 \cdot 6H2O}[/latex]
- [latex]\ce{MoS2}[/latex]
- Each of the following compounds contains a metal that can exhibit more than one ionic charge. Name these compounds:
- [latex]\ce{NiCO3}[/latex]
- [latex]\ce{MoO3}[/latex]
- [latex]\ce{Co(NO3)2}[/latex]
- [latex]\ce{V2O5 }[/latex]
- [latex]\ce{MnO2 }[/latex]
- [latex]\ce{Fe2O3 }[/latex]
- The following ionic compounds are found in common household products. Write the formulas for each compound:
- titanium(IV) oxide
- ammonium nitrate
- sodium bisulfate (the common name for sodium hydrogen sulfate)
- The following ionic compounds are found in common household products. Name each of the compounds:
- [latex]\ce{Ca(H2PO4)2}[/latex]
- [latex]\ce{FeSO4 }[/latex]
- [latex]\ce{CaCO3 }[/latex]
- [latex]\ce{MgO }[/latex]
- [latex]\ce{NaNO2 }[/latex]
- [latex]\ce{KI }[/latex]
- What are the IUPAC names of the following compounds?
- manganese dioxide
- mercurous chloride ([latex]\ce{Hg2Cl2}[/latex])
- ferric nitrate [latex]\ce{Fe(NO3)3}[/latex]
- titanium tetrachloride
- cupric bromide ([latex]\ce{CuBr2}[/latex])
Show Selected Solutions
- The solutions are as follows:
- beryllium chloride
- hydrogen bromide
- aluminum fluoride
- The solutions are as follows:
- [latex]\ce{CaCl2}[/latex]
- [latex]\ce{HF}[/latex]
- [latex]\ce{GaP}[/latex]
- [latex]\ce{AlBr3}[/latex]
- [latex]\ce{(NH4)2SO4}[/latex]
- The solutions are as follows:
- [latex]\ce{Ag2S}[/latex]
- [latex]\ce{AlF \cdot 3H2O}[/latex]
- [latex]\ce{SiO2}[/latex]
- The solutions are as follows:
- titanium(IV) chloride
- cobalt(II) chloride hexahydrate
- molybdenum(IV) sulfide
- The answers are as follows:
- [latex]\ce{TiO2}[/latex]
- [latex]\ce{NH4NO3}[/latex]
- [latex]\ce{NaHSO4}[/latex]
- The answers are as follows:
- manganese(IV) oxide
- mercury(I) chloride
- iron(III) nitrate
- titanium(IV) chloride
- copper(II) bromide
5.5 Formula mass [Go to section 5.5]
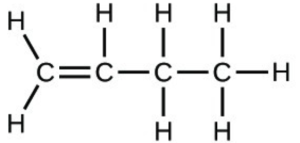
- Determine the molecular mass of the following compounds:
- [latex]\ce{H-C#C-H}[/latex]
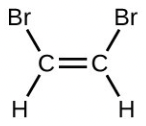
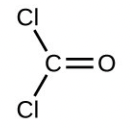
- Calculate the molecular or formula mass of each of the following:
- [latex]\ce{P4}[/latex]
- [latex]\ce{H2O }[/latex]
- [latex]\ce{Ca(NO3)2 }[/latex]
- [latex]\ce{CH3CO2H}[/latex] (acetic acid)
- [latex]\ce{C12H22O11}[/latex] (sucrose, cane sugar)
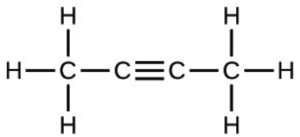
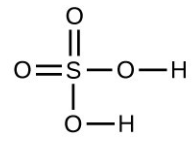
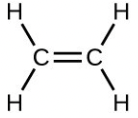
- Which molecule has a molecular mass of 28.05 amu?
- [latex]\ce{H-C#C-H}[/latex]
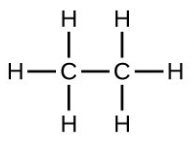
- What is the total mass of hydrogen in each of the molecules?
- [latex]\ce{CH4}[/latex]
- [latex]\ce{CHCl3}[/latex]
- [latex]\ce{C12H10O6}[/latex]
- [latex]\ce{CH3CH2CH2CH2CH3}[/latex]
- Calculate the molecular or formula mass of each of the following:
- [latex]\ce{P4 }[/latex]
- [latex]\ce{H2O}[/latex]
- [latex]\ce{Ca(NO3)2}[/latex]
- [latex]\ce{CH3CO2H}[/latex] (acetic acid)
- [latex]\ce{C12H22O11}[/latex] (sucrose, cane sugar).
- Write a sentence that describes how to determine the number of moles of a compound in a known mass of the compound if we know its molecular formula.
- Compare 1 mole of [latex]\ce{H2}[/latex], 1 mole of [latex]\ce{O2}[/latex], and 1 mole of [latex]\ce{F2}[/latex].
- Which has the largest number of molecules? Explain why.
- Which has the greatest mass? Explain why.
- Which contains the greatest mass of oxygen: 0.75 mol of ethanol ([latex]\ce{C2H5OH}[/latex]), 0.60 mol of formic acid ([latex]\ce{HCO2H}[/latex]), or 1.0 mol of water ([latex]\ce{H2O}[/latex])? Explain why.
- Which contains the greatest number of moles of oxygen atoms: 1 mol of ethanol ([latex]\ce{C2H5OH}[/latex]), 1 mol of formic acid ([latex]\ce{HCO2H}[/latex]), or 1 mol of water ([latex]\ce{H2O}[/latex])? Explain why.
- How are the molecular mass and the molar mass of a compound similar and how are they different?
- Calculate the molar mass of each of the following:
- [latex]\ce{S8}[/latex]
- [latex]\ce{C5H12 }[/latex]
- [latex]\ce{Sc2(SO4)3}[/latex]
- [latex]\ce{CH3COCH3}[/latex] (acetone)
- [latex]\ce{C6H12O6}[/latex] (glucose)
- Calculate the molar mass of each of the following:
- the anesthetic halothane, [latex]\ce{C2HBrClF3}[/latex]
- the herbicide paraquat, [latex]\ce{C12H14N2Cl2}[/latex]
- caffeine, [latex]\ce{C8H10N4O2}[/latex]
- urea, [latex]\ce{CO(NH2)2 }[/latex]
- a typical soap, [latex]\ce{C17H35CO2Na}[/latex]
- Determine the number of moles of compound and the number of moles of each type of atom in each of the following:
- 25.0 g of propylene, [latex]\ce{C3H6}[/latex]
- 3.06 × 10-3 g of the amino acid glycine, [latex]\ce{C2H5NO2}[/latex]
- 25 lb of the herbicide Treflan, [latex]\ce{C13H16N2O4F}[/latex] (1 lb = 454 g)
- 0.125 kg of the insecticide Paris Green, [latex]\ce{Cu4(AsO3)2(CH3CO2)2}[/latex]
- 325 mg of aspirin, [latex]\ce{C6H4(CO2H)(CO2CH3)}[/latex]
- Determine the mass of each of the following:
- 0.0146 mol [latex]\ce{KOH}[/latex]
- 10.2 mol ethane, [latex]\ce{C2H6}[/latex]
- 1.6 × 10-3 mol [latex]\ce{Na2SO4}[/latex]
- 6.854 × 103 mol glucose, [latex]\ce{C6H12O6}[/latex]
- 2.86 mol [latex]\ce{Co(NH3)6Cl3}[/latex]
- Determine the number of moles of the compound and determine the number of moles of each type of atom in each of the following:
- 2.12 g of potassium bromide, [latex]\ce{KBr}[/latex]
- 0.1488 g of phosphoric acid, [latex]\ce{H3PO4}[/latex]
- 23 kg of calcium carbonate, [latex]\ce{CaCO3}[/latex]
- 78.452 g of aluminum sulfate, [latex]\ce{Al2(SO4)3}[/latex]
- 0.1250 mg of caffeine, [latex]\ce{C8H10N4O2}[/latex]
- The approximate minimum daily dietary requirement of the amino acid leucine, [latex]\ce{C6H13NO2}[/latex], is 1.1 g. What is this requirement in moles?
- Determine the mass in grams of each of the following:
- 0.600 mol of oxygen atoms
- 0.600 mol of oxygen molecules, [latex]\ce{O2}[/latex]
- 0.600 mol of ozone molecules, [latex]\ce{O3}[/latex]
- A 55-kg woman has 7.5 × 10-3 mol of hemoglobin (molar mass = 64,456 g/mol) in her blood. How many hemoglobin molecules is this? What is this quantity in grams?
- Determine the number of atoms and the mass of zirconium, silicon, and oxygen found in 0.3384 mol of zircon, [latex]\ce{ZrSiO4}[/latex], a semiprecious stone.
- Determine which of the following contains the greatest mass of aluminum: 122 g of [latex]\ce{AlPO4}[/latex], 266 g of [latex]\ce{A12C16}[/latex], or 225 g of [latex]\ce{A12S3}[/latex].
- Diamond is one form of elemental carbon. An engagement ring contains a diamond weighing 1.25 carats (1 carat = 200 mg). How many atoms are present in the diamond?
- The Cullinan diamond was the largest natural diamond ever found (January 25, 1905). It weighed 3104 carats (1 carat = 200 mg). How many carbon atoms were present in the stone
- One 55-gram serving of a particular cereal supplies 270 mg of sodium, 11% of the recommended daily allowance. How many moles and atoms of sodium are in the recommended daily allowance?
- A certain nut crunch cereal contains 11.0 grams of sugar (sucrose, [latex]\ce{C12H22O11}[/latex]) per serving size of 60.0 grams. How many servings of this cereal must be eaten to consume 0.0278 moles of sugar?
- A tube of toothpaste contains 0.76 g of sodium monofluorophosphate ([latex]\ce{Na2PO3F}[/latex]) in 100 mL
- What mass of fluorine atoms in mg was present?
- How many fluorine atoms were present?
- Which of the following represents the least number of molecules?
- 20.0 g of [latex]\ce{H2O}[/latex] (18.02 g/mol)
- 77.0 g of [latex]\ce{CH4}[/latex] (16.06 g/mol)
- 68.0 g of [latex]\ce{CaH2}[/latex] (42.09 g/mol)
- 100.0 g of [latex]\ce{N2O}[/latex] (44.02 g/mol)
- 84.0 g of [latex]\ce{HF}[/latex] (20.01 g/mol)
- Determine which of the following contains the greatest mass of hydrogen: 1 mol of [latex]\ce{CH4}[/latex], 0.6 mol of [latex]\ce{C6H6}[/latex], or 0.4 mol of [latex]\ce{C3H8}[/latex].
- Calculate the empirical or molecular formula mass and the molar mass of each of the following minerals:
- limestone, [latex]\ce{CaCO3}[/latex]
- halite, [latex]\ce{NaCl}[/latex]
- beryl, [latex]\ce{Be3Al2Si6O13}[/latex]
- malachite, [latex]\ce{Cu2(OH)2CO3}[/latex]
- turquoise, [latex]\ce{CuAl6(PO4)4(OH)8(H2O)4}[/latex]
Show Selected Solutions
- The answers are as follows:
- 123.896 amu
- 18.015 amu
- 164.086 amu
- 60.052 amu
- 342.297 amu
- The answers are as follows:
- 4.032 amu
- 1.008 amu
- 9.921 amu
- 12.096 amu
- Use the atomic weights from the periodic table to determine the molar mass of the compound and divide the mass by the molar mass to determine the number of moles.
- Water ([latex]\ce{H2O}[/latex]) contains the greatest mass because in one mole of water, there is one mole of oxygen with a mass of 15.999 g.
- Molecular mass is synonymous with formula mass and represents the mass of a covalent substance in amu. Molar mass represents the mass of one mole of any compound.
- The answers are as follows:
- 127.4 g/mol
- 257.1 g/mol
- 194.2 g/mol
- 60.01 g/mol
- 306.4 g/mol
- The answers are as follows:
- 6.88 g
- 307 g
- 0.23 g
- 1.23 x 106 g
- 764 g
- 8.4 × 10-3 mol
- 4.5 x 1021 molecules, 4.8 x 102 g
- 266 g of [latex]\ce{A12C16}[/latex]
- 3.113 × 1025 carbon atoms
- 0.864 servings
- 20.0 g of [latex]\ce{H2O}[/latex]
- The answers are as follows:
- [latex]\ce{CaCO3}[/latex]
[latex]\begin{array}{lcccr} \ce{1Ca} & = & 1 × 40.078 & = & 40.078 \text{ g mol}^{-1} \\ 1\ce{C} & = & 1 × 12.011 & = & 12.011 \text{ g mol}^{-1} \\ 3\ce{O} & = & 3 × 15.9994 & = & \underline{47.9982 \text{ g mol}}^{-1} \\ & & & = & 100.087 \text{ g mol}^{-1} \\ \end{array}[/latex] - [latex]\ce{NaCl}[/latex]
[latex]\begin{array}{lcccr} \ce{1Na} & = & 1 × 22.989768 & = & 22.989768 \text{ g mol}^{-1} \\ 1\ce{Cl} & = & 1 × 35.4527 & = & \underline{35.4527 \text{ g mol}}^{-1} \\ & & & = & 58.4425 \text{ g mol}^{-1} \\ \end{array}[/latex] - [latex]\ce{Be3Al2Si6O_{18}}[/latex]
[latex]\begin{array}{lcccr} 3\ce{Be} & = & 3 × 9.01218 & = & 27.03654 \text{ g mol}^{-1} \\ 2\ce{Al} & = & 2 × 26.98154 & = & 53.96308 \text{ g mol}^{-1} \\ 6\ce{Si} & = & 6 × 28.0855 & = & 168.513 \text{ g mol}^{-1} \\ 18\ce{O} & = & 18 × 15.9994 & = & \underline{287.9892 \text{ g mol}}^{-1} \\ & & & = & 537.502 \text{ g mol}^{-1} \\ \end{array}[/latex] - [latex]\ce{Cu2(OH)2CO3}[/latex]
[latex]\begin{array}{lcccr} 2\ce{C} & = & 2 × 63.546 & = & 127.092 \text{ g mol}^{-1} \\ 5\ce{O} & = & 5 × 15.9994 & = & 79.997 \text{ g mol}^{-1} \\ 2\ce{H} & = & 2 × 1.00794 & = & 2.01588 \text{ g mol}^{-1} \\ 1\ce{H} & = & 1 × 12.011 & = & \underline{12.011 \text{ g mol}}^{-1} \\ & & & = & 221.116 \text{ g mol}^{-1} \\ \end{array}[/latex] - [latex]\ce{CuAl6(PO4)4(OH)8(H2O)4}[/latex]
[latex]\begin{array}{lcccr} 1\ce{Cu} & = & 1 × 63.546 & = & 63.546 \text{ g mol}^{-1} \\ 6\ce{Al} & = & 6 × 26.98154 & = & 161.88924 \text{ g mol}^{-1} \\ 4\ce{P} & = & 4 × 30.9737624 & = & 123.89505 \text{ g mol}^{-1} \\ 28\ce{O} & = & 28 × 15.9994 & = & 447.9832 \text{ g mol}^{-1} \\ 16\ce{H} & = & 16 × 1.00794 & = & \underline{16.12704 \text{ g mol}}^{-1} \\ & & & = & 813.441 \text{ g mol}^{-1} \\ \end{array}[/latex]
- [latex]\ce{CaCO3}[/latex]
5.6 Chemical Formulas [Go to section 5.6]
- Write the empirical formulas for the following compounds:
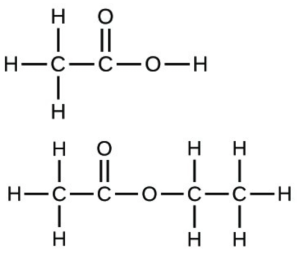
- Determine the empirical formulas for the following compounds:
- acetic acid, [latex]\ce{C2H4O2}[/latex]
- citric acid, [latex]\ce{C6H8O7 }[/latex]
- hydrazine, [latex]\ce{N2H4}[/latex]
- nicotine, [latex]\ce{C10H14N2}[/latex]
- butane, [latex]\ce{C4H10}[/latex]
5.7 Determining Empirical and Molecular Formulas [Go to section 5.7]
- Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition: 28.03% [latex]\ce{Mg}[/latex], 21.60% [latex], 1.16% [latex\ce{H}[/latex], and 49.21% [latex]\ce{O}[/latex]. The molar mass for chrysotile is 520.8 g/mol.
- Polymers are large molecules composed of simple units repeated many times. Thus, they often have relatively simple empirical formulas. Calculate the empirical formulas of the following polymers:
- Lucite (Plexiglas); 59.9% [latex]\ce{C}[/latex], 8.06% [latex]\ce{H}[/latex], 32.0% [latex]\ce{O}[/latex]
- Saran; 24.8% [latex]\ce{C}[/latex], 2.0% [latex]\ce{H}[/latex], 73.1% [latex]\ce{Cl}[/latex]
- polyethylene; 86% [latex]\ce{C}[/latex], 14% [latex]\ce{H}[/latex]
- polystyrene; 92.3% [latex]\ce{C}[/latex], 7.7% [latex]\ce{H}[/latex]
- Orlon; 67.9% [latex]\ce{C}[/latex], 5.70% [latex]\ce{H}[/latex], 26.4% [latex]\ce{N}[/latex]
- Calculate the following to four significant figures:
- the percent composition of ammonia, [latex]\ce{NH3}[/latex]
- the percent composition of photographic fixer solution (“hypo”), [latex]\ce{Na2S2O3}[/latex]
- the percent of calcium ion in [latex]\ce{Ca3(PO4)2}[/latex]
- What information is needed to determine the molecular formula of a compound from the empirical formula?
- A major textile dye manufacturer developed a new yellow dye. The dye has a percent composition of 75.95% [latex]\ce{C}[/latex], 17.72% [latex]\ce{N}[/latex], and 6.33% [latex]\ce{H}[/latex] by mass with a molar mass of about 240 g/mol. Determine the molecular formula of the dye.
- Determine the empirical formulas for compounds with the following percent compositions:
- 43.6% phosphorus and 56.4% oxygen
- 28.7% [latex]\ce{K}[/latex], 1.5% [latex]\ce{H}[/latex], 22.8% [latex]\ce{P}[/latex], and 47.0% [latex]\ce{O}[/latex]
- Explain why the symbol for an atom of the element oxygen and the formula for a molecule of oxygen differ.
- Dichloroethane, a compound that is often used for dry cleaning, contains carbon, hydrogen, and chlorine. It has a molar mass of 99 g/mol. Analysis of a sample shows that it contains 24.3% carbon and 4.1% hydrogen. What is its molecular formula?
- Explain why the symbol for the element sulfur and the formula for a molecule of sulfur differ.
- Determine the following to four significant figures:
- the percent composition of hydrazoic acid, [latex]\ce{HN3}[/latex]
- the percent composition of TNT, [latex]\ce{C6H2(CH3)(NO2)3 }[/latex]
- the percent of [latex]\ce{SO4^{2-}}[/latex]in [latex]\ce{Al2(SO4)3}[/latex]
- Write the molecular and empirical formulas of the following compounds:
- Determine the percent water in [latex]\ce{CuSO4 \cdot 5H2O}[/latex] to three significant figures.
- Determine the empirical formulas for compounds with the following percent compositions:
- 15.8% carbon and 84.2% sulfur
- 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen
- Polymers are large molecules composed of simple units repeated many times. Thus, they often have relatively simple empirical formulas. Calculate the empirical formulas of the following polymers:
- Lucite (Plexiglas); 59.9% [latex]\ce{C}[/latex], 8.06% [latex]\ce{H}[/latex], 32.0% [latex]\ce{O}[/latex]
- Saran; 24.8% [latex]\ce{C}[/latex], 2.0% [latex]\ce{H}[/latex], 73.1% [latex]\ce{Cl}[/latex]
- polyethylene; 86% [latex]\ce{C}[/latex], 14% [latex]\ce{H}[/latex]
- polystyrene; 92.3% [latex]\ce{C}[/latex], 7.7% [latex]\ce{H}[/latex]
- Orlon; 67.9% [latex]\ce{C}[/latex], 5.70% [latex]\ce{H}[/latex], 26.4% [latex]\ce{N}[/latex]
- What information is needed to determine the molecular formula of a compound from the empirical formula?
Show Selected Solutions
- The answers are as follows:
- [latex]\ce{C5H8O2}[/latex]
- [latex]\ce{CHCl}[/latex]
- [latex]\ce{CH2}[/latex]
- [latex]\ce{CH}[/latex]
- [latex]\ce{C3H3N}[/latex]
- After determining the empirical formula, additional information such as the molar mass, or the moles of an element per mole of the compound, must be given.
- The answers are as follows:
- [latex]\ce{P2O5}[/latex]
- [latex]\ce{KH2PO4}[/latex]
- [latex]\ce{C2H4Cl2}[/latex]
- The answers are as follows:
- % [latex]\ce{H}[/latex] = 2.34% ; %[latex]\ce{N}[/latex] = 97.66%
- % [latex]\ce{C}[/latex] = 37.01% ; %[latex]\ce{H}[/latex] = 2.219%; %[latex]\ce{O}[/latex] = 42.26%; %[latex]\ce{N}[/latex]= 18.50%
- % [latex]\ce{SO4^{-2}}[/latex] = 84.23%
- 36.1%
- [latex]\ce{C2H4Cl2}[/latex]
5.8 Writing and Balancing Chemical Equations [Go to section 5.8]
- Balance the following equations:
- [latex]\ce{Ag}(s) + \ce{H2S}(g) + \ce{O2}(g) \longrightarrow \ce{Ag2S}(s) + \ce{H2O}(l)[/latex]
- [latex]\ce{P4}(s) + \ce{O2}(g) \longrightarrow \ce{P4O_{10}}(s)[/latex]
- [latex]\ce{Pb}(s) + \ce{H2O}(l) + \ce{O2}(g) \longrightarrow \ce{pb(OH)2}(s)[/latex]
- [latex]\ce{Fe}(s) + \ce{H2O}(l) \longrightarrow \ce{Fe3O4}(s) + \ce{H2}(g)[/latex]
- [latex]\ce{Sc2O3}(s) + \ce{SO3}(l) \longrightarrow \ce{Sc2(SO4)3}(s)[/latex]
- [latex]\ce{Ca3(PO4)2}(aq) + \ce{H3PO4}(aq) \longrightarrow \ce{Ca(H2PO4)2}(aq)[/latex]
- [latex]\ce{Al}(s) + \ce{H2SO4}(aq) \longrightarrow \ce{Al2(SO4)3} + \ce{H2}(g)[/latex]
- [latex]\ce{TiCl4}(s) + \ce{H2O}(g) \longrightarrow \ce{TiO2}(s) + \ce{HCl}(g)[/latex]
Show Selected Solutions
- The answers are as follows:
- [latex]4\ce{Ag}(s) + 2\ce{H2S}(g) + \ce{O2}(g) \longrightarrow 2\ce{Ag2S}(s) + 2\ce{H2O}(l)[/latex]
- [latex]\ce{P4}(s) + 5\ce{O2}(g) \longrightarrow \ce{P4O_{10}}(s)[/latex]
- [latex]2\ce{Pb}(s) + 2\ce{H2O}(l) + \ce{O2}(g) \longrightarrow 2\ce{pb(OH)2}(s)[/latex]
- [latex]3\ce{Fe}(s) + 4\ce{H2O}(l) \longrightarrow \ce{Fe3O4}(s) + 4\ce{H2}(g)[/latex]
- [latex]\ce{Sc2O3}(s) + 3\ce{SO3}(l) \longrightarrow \ce{Sc2(SO4)3}(s)[/latex]
- [latex]\ce{Ca3(PO4)2}(aq) + 4\ce{H3PO4}(aq) \longrightarrow 3\ce{Ca(H2PO4)2}(aq)[/latex]
- [latex]2\ce{Al}(s) + 3\ce{H2SO4}(aq) \longrightarrow \ce{Al2(SO4)3} + 3\ce{H2}(g)[/latex]
- [latex]\ce{TiCl4}(s) + 2\ce{H2O}(g) \longrightarrow \ce{TiO2}(s) + 4\ce{HCl}(g)[/latex]