Chapter 4: Periodic Properties of the Elements
4.5 Ionic Radii and Isoelectronic Series
Link:
https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_A_Molecular_Approach_(Tro)/08%3A_Periodic_Properties_of_the_Elements/8.06%3A_Periodic_Trends_in_the_Size_of_Atoms_and_Effective_Nuclear_Charge
Learning Objectives
- To predict relative ionic sizes within an isoelectronic series.
Ionic Radii and Isoelectronic Series
An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach themselves to neutral atoms to form a negative one (anion). The designations cation or anion come from the early experiments with electricity which found that positively charged particles were attracted to the negative pole of a battery, the cathode, while negatively charged ones were attracted to the positive pole, the anode.
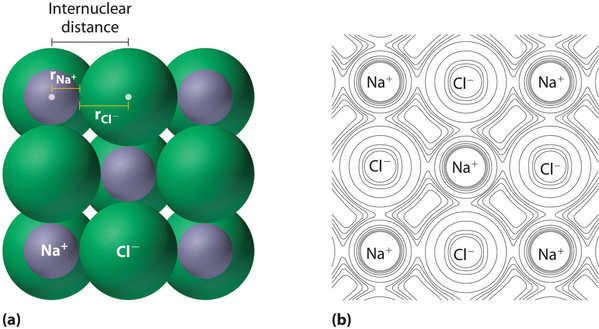
Ionic compounds consist of regular repeating arrays of alternating positively charged cations and negatively charges anions. Although it is not possible to measure an ionic radius directly for the same reason it is not possible to directly measure an atom’s radius, it is possible to measure the distance between the nuclei of a cation and an adjacent anion in an ionic compound to determine the ionic radius (the radius of a cation or anion) of one or both. As illustrated in Figure 4.5.1, the internuclear distance corresponds to the sum of the radii of the cation and anion. A variety of methods have been developed to divide the experimentally measured distance proportionally between the smaller cation and larger anion. These methods produce sets of ionic radii that are internally consistent from one ionic compound to another, although each method gives slightly different values. For example, the radius of the [latex]\ce{Na+}[/latex] ion is essentially the same in [latex]\ce{NaCl}[/latex] and [latex]\ce{Na_2S}[/latex], as long as the same method is used to measure it. Thus despite minor differences due to methodology, certain trends can be observed.
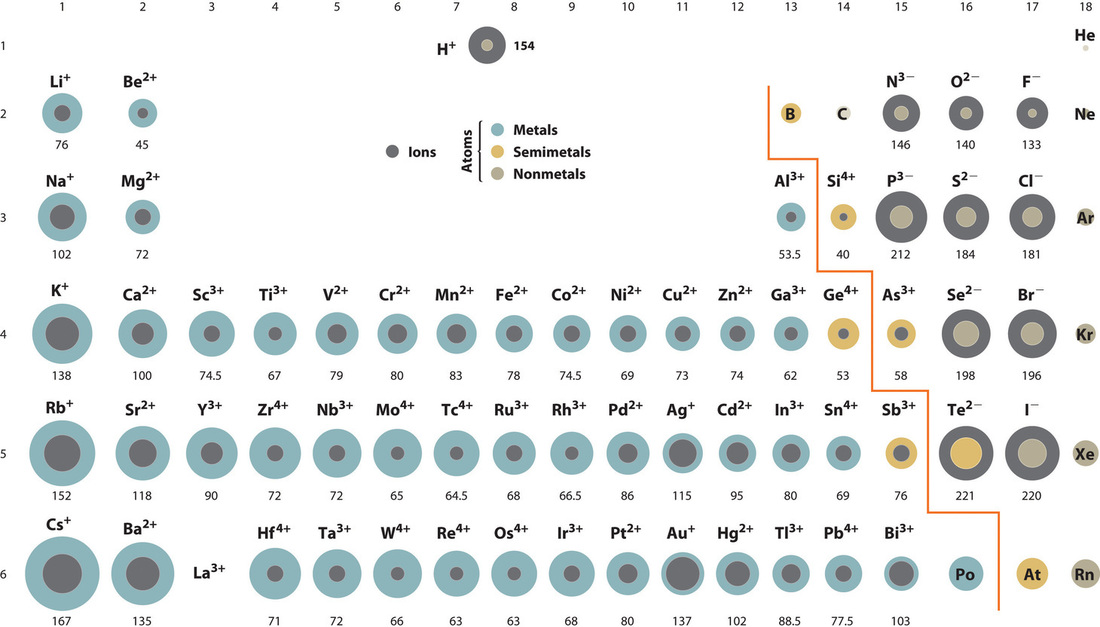
A comparison of ionic radii with atomic radii (Figure 4.5.1) shows that a cation, having lost an electron, is always smaller than its parent neutral atom, and an anion, having gained an electron, is always larger than the parent neutral atom. When one or more electrons is removed from a neutral atom, two things happen: (1) repulsions between electrons in the same principal shell decrease because fewer electrons are present, and (2) the effective nuclear charge felt by the remaining electrons increases because there are fewer electrons to shield one another from the nucleus. Consequently, the size of the region of space occupied by electrons decreases and the ion shrinks (compare [latex]\ce{Li}[/latex] at 167 pm with [latex]\ce{Li+}[/latex] at 76 pm). If different numbers of electrons can be removed to produce ions with different charges, the ion with the greatest positive charge is the smallest (compare [latex]\ce{Fe^2+}[/latex] at 78 pm with [latex]\ce{Fe{3+}}[/latex] at 64.5 pm). Conversely, adding one or more electrons to a neutral atom causes electron–electron repulsions to increase and the effective nuclear charge to decrease, so the size of the probability region increases and the ion expands (compare [latex]\ce{F}[/latex] at 42 pm with [latex]\ce{F-}[/latex] at 133 pm).
Because most elements form either a cation or an anion but not both, there are few opportunities to compare the sizes of a cation and an anion derived from the same neutral atom. A few compounds of sodium, however, contain the [latex]\ce{Na-}[/latex] ion, allowing comparison of its size with that of the far more familiar [latex]\ce{Na+}[/latex] ion, which is found in many compounds. The radius of sodium in each of its three known oxidation states is given in Table 4.5.1. All three species have a nuclear charge of +11, but they contain 10 ([latex]\ce{Na+}[/latex]), 11 ([latex]\ce{Na^0}[/latex]), and 12 ([latex]\ce{Na−}[/latex]) electrons. The [latex]\ce{Na+}[/latex] ion is significantly smaller than the neutral [latex]\ce{Na}[/latex] atom because the 3s1 electron has been removed to give a closed shell with n = 2. The [latex]\ce{Na−}[/latex] ion is larger than the parent [latex]\ce{Na}[/latex] atom because the additional electron produces a 3s2 valence electron configuration, while the nuclear charge remains the same.
Ionic radii follow the same vertical trend as atomic radii; that is, for ions with the same charge, the ionic radius increases going down a column. The reason is the same as for atomic radii: shielding by filled inner shells produces little change in the effective nuclear charge felt by the outermost electrons. Again, principal shells with larger values of n lie at successively greater distances from the nucleus.
Because elements in different columns tend to form ions with different charges, it is not possible to compare ions of the same charge across a row of the periodic table. Instead, elements that are next to each other tend to form ions with the same number of electrons but with different overall charges because of their different atomic numbers. Such a set of species is known as an isoelectronic series. For example, the isoelectronic series of species with the neon closed-shell configuration (1s22s22p6) is shown in Table 4.5.2.
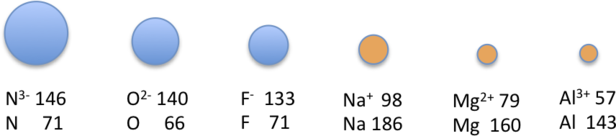
The sizes of the ions in this series decrease smoothly from [latex]\ce{N^3-}[/latex] to [latex]\ce{Al^3+}[/latex]. All six of the ions contain 10 electrons in the 1s, 2s, and 2p orbitals, but the nuclear charge varies from +7 ([latex]\ce{N}[/latex]) to [+13 ([latex]\ce{Al}[/latex]). As the positive charge of the nucleus increases while the number of electrons remains the same, there is a greater electrostatic attraction between the electrons and the nucleus, which causes a decrease in radius. Consequently, the ion with the greatest nuclear charge ([latex]\ce{Al^3+}[/latex]) is the smallest, and the ion with the smallest nuclear charge ([latex]\ce{N^3-}[/latex]) is the largest. The neon atom in this isoelectronic series is not listed in Table 4.5.2, because neon forms no covalent or ionic compounds and hence its radius is difficult to measure.
Example 4.5.1: Trends in Ionic Radii
Based on their positions in the periodic table, arrange these ions in order of increasing radius: [latex]\ce{Cl-}[/latex], [latex]\ce{K+}[/latex], [latex]\ce{S^2-}[/latex], and [latex]\ce{Se^2-}[/latex].
Given: four ions
Asked for: order by increasing radius
Strategy:
- Determine which ions form an isoelectronic series. Of those ions, predict their relative sizes based on their nuclear charges. For ions that do not form an isoelectronic series, locate their positions in the periodic table.
- Determine the relative sizes of the ions based on their principal quantum numbers n and their locations within a row.
Solution:
1 We see that [latex]\ce{S}[/latex] and [latex]\ce{Cl}[/latex] are at the right of the third row, while [latex]\ce{K}[/latex] and [latex]\ce{Se}[/latex] are at the far left and right ends of the fourth row, respectively. [latex]\ce{K+}[/latex], [latex]\ce{Cl-}[/latex], and [latex]\ce{S^2-}[/latex] form an isoelectronic series with the [latex]\ce{[Ar]}[/latex] closed-shell electron configuration; that is, all three ions contain 18 electrons but have different nuclear charges. Because [latex]\ce{K+}[/latex] has the greatest nuclear charge (Z = 19), its radius is smallest, and [latex]\ce{S^2-}[/latex] with Z = 16 has the largest radius. Because selenium is directly below sulfur, we expect the [latex]\ce{Se^2-}[/latex] ion to be even larger than [latex]\ce{S^2-}[/latex].
2 The order must therefore be [latex]\ce{K+}[/latex] < [latex]\ce{Cl-}[/latex] < [latex]\ce{S^2-}[/latex] < [latex]\ce{Se^2-}[/latex].
Check Your Learning
Key Concepts and Summary
The ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in electron–electron repulsions, and the trends in ionic radius parallel those in atomic size. A comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an isoelectronic series, shows a clear correlation between increasing nuclear charge and decreasing size.
Glossary
Ionic radius: the radius of a cation or anion of one or both
Isoelectronic series: ions with the same number of electrons but with different overall charges because of their different atomic numbers
Contributors and Attributions
-
Modified by Joshua Halpern (Howard University)
Licenses and Attributions (Click to expand)
CC licensed content, Shared previously
- N/A. Provided by: N/A. Located at: N/A. License: CC BY: Attribution. License Terms: N/A

