Chapter 4: Periodic Properties of the Elements
Chapter 4 Practice
4.1 Electronic Structure of Atoms (Electron Configurations) [Go to section 4.1]
- Read the labels of several commercial products and identify monatomic ions of at least six main group elements contained in the products. Write the complete electron configurations of these cations and anions.
- Read the labels of several commercial products and identify monatomic ions of at least four transition elements contained in the products. Write the complete electron configurations of these cations.
- Using complete sub-shell notation (1s22s22p6, and so forth), predict the electron configuration of each of the following atoms:
- [latex]\ce{N}[/latex]
- [latex]\ce{Si}[/latex]
- [latex]\ce{Fe}[/latex]
- [latex]\ce{Te}[/latex]
- [latex]\ce{Tb}[/latex]
- Is 1s22s22p6 the symbol for a macroscopic property or a microscopic property of an element? Explain your answer.
- What additional information do we need to answer the question “Which ion has the electron configuration 1s22s22p63s23p6”?
- Draw the orbital diagram for the valence shell of each of the following atoms:
- [latex]\ce{C}[/latex]
- [latex]\ce{P}[/latex]
- [latex]\ce{V}[/latex]
- [latex]\ce{Sb}[/latex]
- [latex]\ce{Ru}[/latex]
- Which ion with a +1 charge has the electron configuration 1s22s22p63s23p63d104s24p6? Which ion with a –2 charge has this configuration?
- Using complete sub-shell notation (1s22s22p6, and so forth), predict the electron configurations of the following ions.
- [latex]\ce{N^3-}[/latex]
- [latex]\ce{Ca^2+}[/latex]
- [latex]\ce{S-}[/latex]
- [latex]\ce{Cs^2+}[/latex]
- [latex]\ce{Cr^2+}[/latex]
- [latex]\ce{Gd^3+}[/latex]
- Which of the following has two unpaired electrons?
- [latex]\ce{Mg}[/latex]
- [latex]\ce{Si}[/latex]
- [latex]\ce{S}[/latex]
- Both [latex]\ce{Mg}[/latex] and [latex]\ce{S}[/latex]
- Both [latex]\ce{Si}[/latex] and [latex]\ce{S}[/latex]
- Which atom has the electron configuration 1s22s22p63s23p63d74s2?
- Which atom would be expected to have a half-filled 4s sub-shell?
- Which of the following atoms contains only three valence electrons: [latex]\ce{Li}[/latex], [latex]\ce{B}[/latex], [latex]\ce{N}[/latex], [latex]\ce{F}[/latex], [latex]\ce{Ne}[/latex]?
- Thallium was used as a poison in the Agatha Christie mystery story “The Pale Horse.” Thallium has two possible cationic forms, +1 and +3. The +1 compounds are the more stable. Write the electron configuration of the +1 cation of thallium.
- Which atom would be expected to have a half-filled 6p sub-shell?
- Cobalt–60 and iodine–131 are radioactive isotopes commonly used in nuclear medicine. How many protons, neutrons, and electrons are in atoms of these isotopes? Write the complete electron configuration for each isotope.
- In one area of Australia, the cattle did not thrive despite the presence of suitable forage. An investigation showed the cause to be the absence of sufficient cobalt in the soil. Cobalt forms cations in two oxidation states, [latex]\ce{Co^2+}[/latex] and [latex]\ce{Co^3+}[/latex]. Write the electron configuration of the two cations.
- Given the following part of an electron configuration, 3s2, explain which part refers to the number of electrons, the energy level and the sub-level.
- What is the maximum number of electrons that can occupy a single orbital?
- For each of the following electron configurations, name the element:
- ↑↓1s _↑↓2s __↑↓_ _↑↓__ _↑__2p
- __↑↓_ 1s __↑↓_2s __↑↓_ __↑↓_ _↑↓_2p _↑↓__3s __↑↓_ __↑↓_ __↑↓_3p _↑↓__4s _↑__ __↑_ __↑_ _↑_ __↑_3d
- State and explain Hund’s Rule. Write the electron configuration (orbital notation) for nitrogen using this rule.
- Write the electron configurations using noble gas notation for the elements in Group 2 of the periodic table.
- Which of the following atoms or ions has three unpaired electrons?
- What is the electron configuration for the barium atom?
- What is the complete electron configuration of tin?
- Which of the following statements is true?
- The exact location of an electron can be determined if we know its energy.
- An electron in a 2s orbital can have the same n, l, and ml quantum numbers as an electron in a 3s orbital.
- [latex]\ce{Ni}[/latex] has two unpaired electrons in its 3d orbitals.
- In the buildup of atoms, electrons occupy the 4f orbitals before the 6s orbitals.
- Only three quantum numbers are needed to uniquely describe an electron.
- What is the statement that “the lowest energy configuration for an atom is the one having the maximum number of unpaired electrons allowed by the Pauli principle in a particular set of degenerate orbitals” known as?
- An element with the electron configuration [latex]\ce{[Xe]}[/latex] 6s24f145d7 would belong to which class on the periodic table?
- What is the statement that “the lowest energy configuration for an atom is the one having the maximum number of unpaired electrons allowed by the Pauli principle in a particular set of degenerate orbitals” known as?
Show Selected Solutions
- For example, [latex]\ce{Na^+}[/latex]: 1s22s22p6; [latex]\ce{Ca2+}[/latex]: 1s22s22p63s23p6; [latex]\ce{Sn2+}[/latex]: 1s22s22p63s23p63d104s24p64d105s2; [latex]\ce{F^-}[/latex]: 1s22s22p6; [latex]\ce{O^{2-}}[/latex]: 1s22s22p6; [latex]\ce{Cl^-}[/latex]: 1s22s22p63s23p6
- The answers are as follows:
- 1s22s22p3
- 1s22s22p63s23p2
- 1s22s22p63s23p64s23d6
- 1s22s22p63s23p64s23d104p65s24d105p4
- 1s22s22p63s23p64s23d104p65s24d105p66s24f9
- The charge on the ion.
- [latex]\ce{Rb^+}[/latex], [latex]\ce{Se^2-}[/latex]
- Although both (b) and (c) are correct, (e) encompasses both and is the best answer.
- K
- 1s22s22p63s23p63d104s24p64d105s25p66s24f145d10
- [latex]\ce{Co}[/latex] has 27 protons, 27 electrons, and 33 neutrons: 1s22s22p63s23p64s23d7. [latex]\ce{I}[/latex] has 53 protons, 53 electrons, and 78 neutrons: 1s22s22p63s23p63d104s24p64d105s25p5.
- 2 is the number of electrons, n = 3, the principle quantum number gives the energy level of the electron, and s is the sub-level.
- The answers are as follows:
- Flourine ([latex]\ce{F}[/latex])
- Manganese ([latex]\ce{Mn}[/latex])
- [latex]\ce{Be}[/latex]: [latex]\ce{[He]}[/latex] [latex]2s^2\ce{Mg}[/latex]: [latex]\ce{[Ne]}[/latex] [latex]3s^2\ce{Ca}[/latex]: [latex]\ce{[Ar]}[/latex] [latex]4s^2\ce{Sr}[/latex]: [latex]\ce{[Kr]}[/latex] [latex]5s^2\ce{Ba}[/latex]: [latex]\ce{[Xe]}[/latex] [latex]6s^2\ce{Ra}[/latex]: [latex]\ce{[Rn]}[/latex] [latex]7s^2[/latex]
- 1s22s22p63s23p64s23d104p65s24d105p66s2
- c
- Transition element
4.3 Periodic Trends in the Size of Atoms [Go to section 4.3]
- Based on their positions in the periodic table, predict which has the smallest atomic radius: [latex]\ce{Mg, Sr, Si, Cl, I}[/latex].
- Based on their positions in the periodic table, predict which has the largest atomic radius: [latex]\ce{Li, Rb, N, F, I}[/latex].
- Based on their positions in the periodic table, list the following atoms in order of increasing radius: [latex]\ce{Mg, Ca, Rb, Cs}[/latex].
- On the Periodic Table below, draw circles to represent the trend in atomic size for:
- a group
- a period
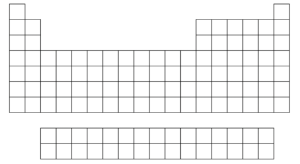
- Which of the following atoms has the largest atomic radius?
4.4 Ionization energy and Electron Affinity [Go to section 4.4]
- Based on their positions in the periodic table, predict which has the smallest first ionization energy: [latex]\ce{Li, Cs, N, F, I}[/latex].
- Based on their positions in the periodic table, rank the following atoms in order of increasing first ionization energy: [latex]\ce{F, Li, N, Rb}[/latex].
- Based on their positions in the periodic table, rank the following atoms or compounds in order of increasing first ionization energy: [latex]\ce{Mg, O, S, Si}[/latex].
- Of the five elements [latex]\ce{Sn, Si, Sb, O, Te}[/latex], which has the most endothermic reaction? (E represents an atom.) What name is given to the energy for the reaction?
[latex]\text{E}(g) \longrightarrow \text{E}^+(g) + \ce{e-}[/latex]
- Of the five elements [latex]\ce{Al, Cl, I, Na, Rb}[/latex], which has the most exothermic reaction? (E represents an atom.) What name is given to the energy for the reaction? (Hint: Note the process depicted does not correspond to electron affinity.) [latex]\text{E}^+(g) + \ce{e-} \longrightarrow \text{E}(g)[/latex]
- Which main group atom would be expected to have the lowest second ionization energy?
4.5 Ionic Radii and Isoelectronic Series [Go to section 4.5]
- List the following ions in order of increasing radius: [latex]\ce{Li^+, Mg^{2+}, Br^-, Te^{2-}}[/latex].
- Which atom and/or ion is (are) isoelectronic with [latex]\ce{Br^+: Se^{2+}, Se, As^-, Kr, Ga^{3+}, Cl^-}[/latex]?
- Which of the following atoms and ions is (are) isoelectronic with [latex]\ce{S^{2+}: Si^{4+}, Cl^{3+}, Ar, As^{3+}, Si, Al^{3+}}[/latex]?
- Compare both the numbers of protons and electrons present in each to rank the following ions in order of increasing radius: [latex]\ce{As^{3-}, Br^-, K^+, Mg^{2+}}[/latex].
- The ionic radii of the ions [latex]\ce{S^{2-}, Cl^-}, \text{and } \ce{K^+}[/latex] are 184, 181, 138 pm respectively. Explain why these ions have different sizes even though they contain the same number of electrons.

