Chapter 3: The Quantum-Mechanical Model of the Atom
Chapter 3 Practice
3.1 Electromagnetic Energy [Go to section 3.1]
- FM–95, an FM radio station, broadcasts at a frequency of 9.51 × 107 s-1 (95.1 MHz). What is the wavelength of these radio waves in meters?
- An FM radio station found at 103.1 on the FM dial broadcasts at a frequency of 1.031 × 108 s-1 (103.1 MHz). What is the wavelength of these radio waves in meters?
- When rubidium ions are heated to a high temperature, two lines are observed in its line spectrum at wavelengths (a) 7.9 × 10-7 m and (b) 4.2 × 10-7 m. What are the frequencies of the two lines? What color do we see when we heat a rubidium compound?
3.2 Quantum Theory [Go to section 3.2]
- A bright violet line occurs at 435.8 nm in the emission spectrum of mercury vapor. What amount of energy, in joules, must be released by an electron in a mercury atom to produce a photon of this light?
- The light produced by a red neon sign is due to the emission of light by excited neon atoms. Qualitatively describe the spectrum produced by passing light from a neon lamp through a prism.
- Heated lithium atoms emit photons of light with an energy of 2.961 10-19 J. Calculate the frequency and wavelength of one of these photons. What is the total energy in 1 mole of these photons? What is the color of the emitted light?
- Light with a wavelength of 614.5 nm looks orange. What is the energy, in joules, per photon of this orange light? What is the energy in eV (1 eV = 1.602 × 10-19 J)?
- A photon of light produced by a surgical laser has an energy of 3.027 × 10-19 J. Calculate the frequency and wavelength of the photon. What is the total energy in 1 mole of photons? What is the color of the emitted light?
- One of the radiographic devices used in a dentist’s office emits an X-ray of wavelength 2.090 × 10-11 m. What is the energy, in joules, and frequency of this X-ray?
- Answer the following questions about a Blu-ray laser:
- The laser on a Blu-ray player has a wavelength of 405 nm. In what region of the electromagnetic spectrum is this radiation? What is its frequency?
- A Blu-ray laser has a power of 5 milliwatts (1 watt = 1 J s-1). How many photons of light are produced by the laser in 1 hour?
- RGB color television and computer displays use cathode ray tubes that produce colors by mixing red, green, and blue light. If we look at the screen with a magnifying glass, we can see individual dots turn on and off as the colors change. Using a spectrum of visible light, determine the approximate wavelength of each of these colors. What is the frequency and energy of a photon of each of these colors?
- Calculate the maximum kinetic energy of an electron ejected by a photon, with a wavelength of 52nm, from a metal with a binding energy of 3.7eV (1eV = 1.602 × 10-19 J).
Show Selected Solutions
- The spectrum consists of colored lines, at least one of which (probably the brightest) is red.
- 2.018 eV
- 9.502 x 10-15 J; 1.434 × 1019 s-1
- See Figure 3.13. Red: 660 nm; 4.54 × 1014 Hz; 3.01 × 10-19 J. Green: 520 nm; 5.77 × 1014 Hz; 3.82 × 10-19 J. Blue: 440 nm; 6.81 × 1014 Hz; 4.51 × 10-19 J. Somewhat different numbers are also possible.
3.3 The Bohr Model [Go to section 3.3]
- What does it mean to say that the energy of the electrons in an atom is quantized?
- Why is the electron in a Bohr hydrogen atom bound less tightly when it has a quantum number of 3 than when it has a quantum number of 1?
- The electron volt (eV) is a convenient unit of energy for expressing atomic-scale energies. It is the amount of energy that an electron gains when subjected to a potential of 1 volt; 1 eV = 1.602 × 10-19 J. Using the Bohr model, determine the energy, in electron volts, of the photon produced when an electron in a hydrogen atom moves from the orbit with n = 5 to the orbit with n = 2. Show your calculations.
- Using the Bohr model, determine the energy, in joules, necessary to ionize a ground-state hydrogen atom. Show your calculations.
- Using the Bohr model, determine the lowest possible energy for the electron in the [latex]\ce{He^+}[/latex] ion
- Using the Bohr model, determine the lowest possible energy, in joules, for the electron in the [latex]\ce{Li^{2+}}[/latex] ion
- Using the Bohr model, determine the energy of an electron with n = 8 in a hydrogen atom.
- Using the Bohr model, determine the energy of an electron with n = 6 in a hydrogen atom.
- What is the radius, in angstroms, of the orbital of an electron with n = 8 in a hydrogen atom?
- How far from the nucleus in angstroms (1 angstrom = 1 × 10-10 m) is the electron in a hydrogen atom if it has an energy of –8.72 × 10-20 J?
- Using the Bohr model, determine the energy in joules of the photon produced when an electron in a [latex]\ce{Li^{2+}}[/latex] ion moves from the orbit with n = 2 to the orbit with n = 1.
- Using the Bohr model, determine the energy in joules of the photon produced when an electron in a [latex]\ce{He^+}[/latex] ion moves from the orbit with n = 5 to the orbit with n = 2.
- Calculate the frequencies, wavelengths, and energies associated with the transition of electrons in the n = 3 orbit of a hydrogen atom to the n = 2 and n = 1 orbits.
Show Selected Solutions
- Quantized energy means that the electrons can possess only certain discrete energy values; values between those quantized values are not permitted.
- 2.856 eV
- -8.716 × 10-18 J
- -3.405 × 10-20 J
- 33.9 Å
- 1.471 × 10-17 J
-
Transition Wavelength (nm) Frequency (s-1) Energy (J) n3→ n2 656 4.57 × 1014 -3.03 × 10-19 n3 → n1 102 2.93 × 1015 -1.94 × 10-18 n2 → n1 121 2.48 × 1015 -1.64 × 10-18
3.4 The Wavelength Nature of Matter [Go to section 3.4]
- Which of the equations from Section 3.4 describe particle-like behavior? Which describe wavelike behavior? Do any involve both types of behavior? Describe the reasons for your choices.
- Calculate the minimum uncertainty in the velocity of an electron that has an uncertainty of 600 pm in its position.
3.5 Quantum Mechanics and The Atom [Go to section 3.5]
- What are the allowed values for each of the four quantum numbers: n, l, ml, and ms?
- Describe the properties of an electron associated with each of the following four quantum numbers: n, l, ml, and ms.
- Answer the following questions:
- Without using quantum numbers, describe the differences between the shells, sub-shells, and orbitals of an atom.
- How do the quantum numbers of the shells, sub-shells, and orbitals of an atom differ?
- Identify the sub-shell in which electrons with the following quantum numbers are found:
- n = 2, l = 1
- n = 4, l = 2
- n = 6, l = 0
- Which of the sub-shells described in Exercise 35 contain degenerate orbitals? How many degenerate orbitals are in each?
- Identify the sub-shell in which electrons with the following quantum numbers are found:
- n = 3, l = 2
- n = 1, l = 0
- n = 4, l = 3
- Which of the sub-shells described in Exercise 37 contain degenerate orbitals? How many degenerate orbitals are in each?
- Write a set of quantum numbers for each of the electrons with an n of 4 in a Se atom.
- How many electrons could be held in the second shell of an atom if the spin quantum number ms could have three values instead of just two? (Hint: Consider the Pauli exclusion principle.)
Show Selected Solutions
- n determines the general range for the value of energy and the probable distances that the electron can be from the nucleus. l determines the shape of the orbital. m1 determines the orientation of the orbitals of the same l value with respect to one another. ms determines the spin of an electron.
- The answers are as follows:
- 2p
- 4d
- 6s
- The answers are follows:
- 3d
- 1s
- 4f
-
n l ml s 4 0 0 +1/2 4 0 0 -1/2 4 1 -1 +1/2 4 1 0 +1/2 4 1 +1 +1/2 4 1 -1 -1/2
3.6 The Shape of Atomic Orbitals [Go to section 3.6]
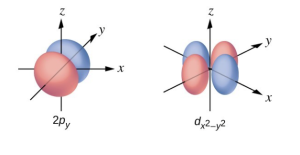
- Sketch the boundary surface of a dx2-y2 and a py orbital. Be sure to show and label the axes.
- Sketch the px and dxz orbitals. Be sure to show and label the coordinates.
- Consider the orbitals shown here in outline.
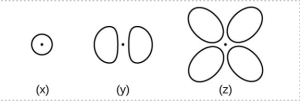
- What is the maximum number of electrons contained in an orbital of type (x)? Of type (y)? Of type (z)?
- How many orbitals of type (x) are found in a shell with n = 2? How many of type (y)? How many of type (z)?
- Write a set of quantum numbers for an electron in an orbital of type (x) in a shell with n = 4. Of an orbital of type (y) in a shell with n = 2. Of an orbital of type (z) in a shell with n = 3.
- What is the smallest possible n value for an orbital of type (x)? Of type (y)? Of type (z)?
- What are the possible l and ml values for an orbital of type (x)? Of type (y)? Of type (z)?