Chapter 7: Advanced Theories of Covalent Bonding
7.1 Molecular Structure and VSEPR Theory
Learning Outcomes
- Predict the structures of small molecules using valence shell electron pair repulsion (VSEPR) theory
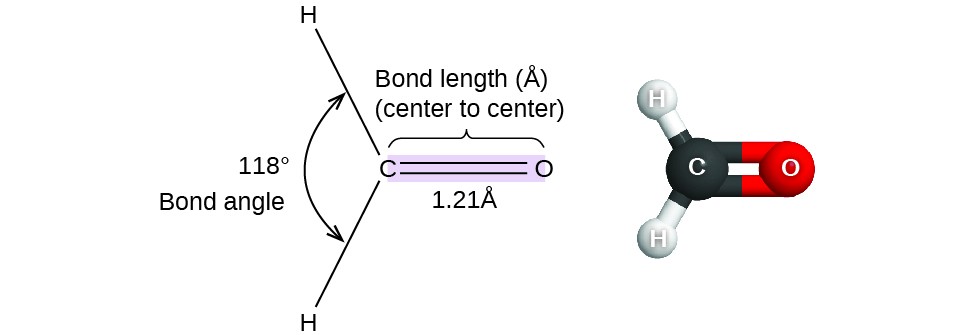
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space (Figure 7.1.1). A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. A bond distance (or bond length) is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Bond distances are measured in Ångstroms (1 Å = 10-10 m) or picometers (1 pm = 10-12 m, 100 pm = 1 Å).
VSEPR Theory
Valence shell electron-pair repulsion theory enables us to predict the molecular structure, including approximate bond angles around a central atom, of a molecule from an examination of the number of bonds and lone electron pairs in its Lewis structure. The VSEPR model assumes that electron pairs in the valence shell of a central atom will adopt an arrangement that minimizes repulsions between these electron pairs by maximizing the distance between them. The electrons in the valence shell of a central atom form either bonding pairs of electrons, located primarily between bonded atoms, or lone pairs. The electrostatic repulsion of these electrons is reduced when the various regions of high electron density assume positions as far from each other as possible.
VSEPR theory predicts the arrangement of electron pairs around each central atom and, usually, the correct arrangement of atoms in a molecule. We should understand, however, that the theory only considers electron-pair repulsions. Other interactions, such as nuclear-nuclear repulsions and nuclear-electron attractions, are also involved in the final arrangement that atoms adopt in a particular molecular structure.

As a simple example of VSEPR theory, let us predict the structure of a gaseous [latex]\ce{BeF2}[/latex] molecule. The Lewis structure of [latex]\ce{BeF2}[/latex] (Figure 7.1.2) shows only two electron pairs around the central beryllium atom. With two bonds and no lone pairs of electrons on the central atom, the bonds are as far apart as possible, and the electrostatic repulsion between these regions of high electron density is reduced to a minimum when they are on opposite sides of the central atom. The bond angle is 180° (Figure 7.1.2). Figure 7.1.3 illustrates this and other electron-pair geometries that minimize the repulsions among regions of high electron density (bonds and/or lone pairs). Two regions of electron density around a central atom in a molecule form a linear geometry; three regions form a trigonal planar geometry; four regions form a tetrahedral geometry; five regions form a trigonal bipyramidal geometry; and six regions form an octahedral geometry.
Figure 7.1.3 The basic electron-pair geometries predicted by VSEPR theory maximize the space around any region of electron density (bonds or lone pairs).
Key Concepts and Summary
VSEPR theory predicts the three-dimensional arrangement of atoms in a molecule. It states that valence electrons will assume an electron-pair geometry that minimizes repulsions between areas of high electron density (bonds and/or lone pairs).
Try It
- Explain why the [latex]\ce{HOH}[/latex] molecule is bent, whereas the HBeH molecule is linear.
- Predict the electron pair geometry of each of the following molecules or ions:
- [latex]\ce{SF6}[/latex]
- [latex]\ce{PCl5}[/latex]
- [latex]\ce{BeH2}[/latex]
Show Selected Solutions
- The placement of the two sets of unpaired electrons in water forces the bonds to assume a tetrahedral arrangement, and the resulting [latex]\ce{HOH}[/latex] molecule is bent. The [latex]\ce{HBeH}[/latex] molecule (in which Be has only two electrons to bond with the two electrons from the hydrogens) must have the electron pairs as far from one another as possible and is therefore linear.
- The electron pair geometry and the molecular structure of each are as follows:
- Number of valence electrons: [latex]\ce{S}[/latex] = 6, [latex]\ce{F}[/latex] = 7 each, total 48. A single line bond represents two electrons:

The total number of electrons used is 48; six bonds are formed and no nonbonded pairs exist. Therefore the molecule includes six regions of electron density and, from the table, the electron geometry is octahedral. Since no lone pairs exist, the electron geometry and molecular structure are the same. - Number of valence electrons: [latex]\ce{P}[/latex] = 5, [latex]\ce{Cl}[/latex] = 7 each, total 40:

The total number of electrons is 40; there are five regions of electron density and, from the table, the geometry is trigonal bipyramid. Since no lone pairs exist on [latex]\ce{P}[/latex], the electron geometry and molecular structure are the same. - Number of valence electrons: [latex]\ce{Be}[/latex] = 2, [latex]\ce{H}[/latex] = 1 each, total 4:

There are only two regions of electron density and they must have a linear arrangement. These regions also correspond to the location of the bonds. Both the electron and molecular structures are linear.
- Number of valence electrons: [latex]\ce{S}[/latex] = 6, [latex]\ce{F}[/latex] = 7 each, total 48. A single line bond represents two electrons:
Glossary
bond angle: angle between any two covalent bonds that share a common atom
bond distance: (also, bond length) distance between the nuclei of two bonded atoms
linear: shape in which two outside groups are placed on opposite sides of a central atom
octahedral: shape in which six outside groups are placed around a central atom such that a three-dimensional shape is generated with four groups forming a square and the other two forming the apex of two pyramids, one above and one below the square plane
tetrahedral: shape in which four outside groups are placed around a central atom such that a three-dimensional shape is generated with four corners and 109.5° angles between each pair and the central atom
trigonal bipyramidal: shape in which five outside groups are placed around a central atom such that three form a flat triangle with 120° angles between each pair and the central atom, and the other two form the apex of two pyramids, one above and one below the triangular plane
trigonal planar: shape in which three outside groups are placed in a flat triangle around a central atom with 120° angles between each pair and the central atom
valence shell electron-pair repulsion theory (VSEPR): theory used to predict the bond angles in a molecule based on positioning regions of high electron density as far apart as possible to minimize electrostatic repulsion
angle between any two covalent bonds that share a common atom
(also, bond length) distance between the nuclei of two bonded atoms
theory used to predict the bond angles in a molecule based on positioning regions of high electron density as far apart as possible to minimize electrostatic repulsion
shape in which two outside groups are placed on opposite sides of a central atom
shape in which three outside groups are placed in a flat triangle around a central atom with 120° angles between each pair and the central atom
shape in which four outside groups are placed around a central atom such that a three-dimensional shape is generated with four corners and 109.5° angles between each pair and the central atom
shape in which five outside groups are placed around a central atom such that three form a flat triangle with 120° angles between each pair and the central atom, and the other two form the apex of two pyramids, one above and one below the triangular plane
shape in which six outside groups are placed around a central atom such that a three-dimensional shape is generated with four groups forming a square and the other two forming the apex of two pyramids, one above and one below the square plane

