Chapter 7: Advanced Theories of Covalent Bonding
Chapter 7 Practice
7.1 Molecular Structure and VSEPR Theory [Go to section 7.1] & 7.2 Electron Pair Geometry versus Molecular Structure [Go to section 7.2]
- Explain why the [latex]\ce{HOH}[/latex] molecule is bent, whereas the [latex]\ce{HBeH}[/latex] molecule is linear.
- What feature of a Lewis structure can be used to tell if a molecule’s (or ion’s) electron-pair geometry and molecular structure will be identical?
- Explain the difference between electron-pair geometry and molecular structure.
- Why is the [latex]\ce{H–N–H}[/latex] angle in [latex]\ce{NH3}[/latex] smaller than the [latex]\ce{H–C–H}[/latex] bond angle in [latex]\ce{CH4}[/latex]? Why is the [latex]\ce{H–N–H}[/latex] angle in [latex]\ce{NI4^+}[/latex] identical to the [latex]\ce{H–C–H}[/latex] bond angle in [latex]\ce{ CH4}[/latex]?
- Explain how a molecule that contains polar bonds can be non-polar.
- As a general rule, MXn molecules (where M represents a central atom and X represents terminal atoms; n = 2 – 5) are polar if there is one or more lone pairs of electrons on M. [latex]\ce{NH3}[/latex] (M = N, X = H, n = 3) is an example. There are two molecular structures with lone pairs that are exceptions to this rule. What are they?
- Predict the electron pair geometry and the molecular structure of each of the following molecules or ions:
- [latex]\ce{SF6}[/latex]
- [latex]\ce{PCl5}[/latex]
- [latex]\ce{BeH2}[/latex]
- [latex]\ce{CH3^+}[/latex]
- Identify the electron pair geometry and the molecular structure of each of the following molecules or ions:
- [latex]\ce{IF6^+}[/latex]
- [latex]\ce{CF4}[/latex]
- [latex]\ce{BF3}[/latex]
- [latex]\ce{SiF5^-}[/latex]
- [latex]\ce{BeCl2}[/latex]
- What are the electron-pair geometry and the molecular structure of each of the following molecules or ions?
- [latex]\ce{ClF5}[/latex]
- [latex]\ce{CIO2^-}[/latex]
- [latex]\ce{TeCl4^{2-}}[/latex]
- [latex]\ce{PCl3}[/latex]
- [latex]\ce{SeF4}[/latex]
- [latex]\ce{PH2^-}[/latex]
- Predict the electron pair geometry and the molecular structure of each of the following ions:
- [latex]\ce{H3O^+}[/latex]
- [latex]\ce{PCl4^-}[/latex]
- [latex]\ce{SnCl3^-}[/latex]
- [latex]\ce{BrCl4^-}[/latex]
- [latex]\ce{ICl3}[/latex]
- [latex]\ce{XeF4}[/latex]
- [latex]\ce{SF2}[/latex]
- Identify the electron pair geometry and the molecular structure of each of the following molecules:
- [latex]\ce{ClNO}[/latex] ([latex]\ce{N}[/latex] is the central atom)
- [latex]\ce{CS2}[/latex]
- [latex]\ce{Cl2CO}[/latex] ([latex]\ce{C}[/latex] is the central atom)
- [latex]\ce{Cl2SO}[/latex] ([latex]\ce{S}[/latex] is the central atom)
- [latex]\ce{SO2F2}[/latex] ([latex]\ce{S}[/latex] is the central atom)
- [latex]\ce{XeO2F2}[/latex] ([latex]\ce{Xe}[/latex] is the central atom)
- [latex]\ce{ClOF2^+}[/latex] ([latex]\ce{Cl}[/latex] is the central atom)
- Predict the electron pair geometry and the molecular structure of each of the following:
- [latex]\ce{IOF5}[/latex] ([latex]\ce{I}[/latex] is the central atom)
- POCl3 ([latex]\ce{P}[/latex] is the central atom)
- [latex]\ce{Cl2SeO}[/latex] ([latex]\ce{Se}[/latex] is the central atom)
- [latex]\ce{ClSO^+}[/latex] ([latex]\ce{S}[/latex] is the central atom)
- [latex]\ce{F^2SO}[/latex] ([latex]\ce{S}[/latex] is the central atom)
- [latex]\ce{NO2^-}[/latex]
- [latex]\ce{SiO4^{4-}}[/latex]
- Which of the following molecules and ions contain polar bonds? Which of these molecules and ions have dipole moments?
- [latex]\ce{ClF5}[/latex]
- [latex]\ce{ClO2^-}[/latex]
- [latex]\ce{TeCl4^{2-}}[/latex]
- [latex]\ce{PCl3}[/latex]
- [latex]\ce{SeF4}[/latex]
- [latex]\ce{PH2^-}[/latex]
- [latex]\ce{XeF2}[/latex]
- Which of these molecules and ions contain polar bonds? Which of these molecules and ions have dipole moments?
- [latex]\ce{H3O^+}[/latex]
- [latex]\ce{PCl4^-}[/latex]
- [latex]\ce{SnCl3^-}[/latex]
- [latex]\ce{BrCl4^-}[/latex]
- [latex]\ce{ICl3}[/latex]
- [latex]\ce{XeF4}[/latex]
- [latex]\ce{SF2}[/latex]
- Which of the following molecules have dipole moments?
- [latex]\ce{CS2}[/latex]
- [latex]\ce{SeS2}[/latex]
- [latex]\ce{CCl2F2}[/latex]
- [latex]\ce{PCl3}[/latex] ([latex]\ce{P}[/latex] is the central atom)
- [latex]\ce{ClNO}[/latex] ([latex]\ce{N}[/latex] is the central atom)
- Identify the molecules with a dipole moment:
- [latex]\ce{SF4}[/latex]
- [latex]\ce{CF4}[/latex]
- [latex]\ce{Cl2CCBr2}[/latex]
- [latex]\ce{CH3Cl}[/latex]
- [latex]\ce{H2CO}[/latex]
- The molecule [latex]\ce{XF3}[/latex] has a dipole moment. Is [latex]\ce{X}[/latex] boron or phosphorus?
- The molecule [latex]\ce{XCl2}[/latex] has a dipole moment. Is [latex]\ce{X}[/latex] beryllium or sulfur?
- Is the [latex]\ce{Cl2BBCl2}[/latex] molecule polar or non-polar?
- There are three possible structures for [latex]\ce{PCl2F3}[/latex] with phosphorus as the central atom. Draw them and discuss how measurements of dipole moments could help distinguish among them.
- Describe the molecular structure around the indicated atom or atoms:
- the sulfur atom in sulfuric acid, [latex]\ce{H2SO4 [(HO)2SO2]}[/latex]
- the chlorine atom in chloric acid, [latex]\ce{HClO3 [HOClO2]}[/latex]
- the oxygen atom in hydrogen peroxide, [latex]\ce{HOOH}[/latex]
- the nitrogen atom in nitric acid, [latex]\ce{HNO3 [HONO2]}[/latex]
- the oxygen atom in the [latex]\ce{OH}[/latex] group in nitric acid, [latex]\ce{HNO3 [HONO2]}[/latex]
- the central oxygen atom in the ozone molecule, [latex]\ce{O3}[/latex]
- each of the carbon atoms in propyne, [latex]\ce{CH3CCH}[/latex]
- the carbon atom in Freon, [latex]\ce{CCl2F2}[/latex]
- each of the carbon atoms in allene, [latex]\ce{H2CCCH2}[/latex]
- Draw the Lewis structures and predict the shape of each compound or ion:
- [latex]\ce{CO2}[/latex]
- [latex]\ce{NO2^-}[/latex]
- [latex]\ce{SO3}[/latex]
- [latex]\ce{SO3^{2-}}[/latex]
- A molecule with the formula AB2, in which A and B represent different atoms, could have one of three different shapes. Sketch and name the three different shapes that this molecule might have. Give an example of a molecule or ion for each shape.
- A molecule with the formula AB3, in which A and B represent different atoms, could have one of three different shapes. Sketch and name the three different shapes that this molecule might have. Give an example of a molecule or ion that has each shape.
- Draw the Lewis electron dot structures for these molecules, including resonance structures where appropriate:
- [latex]\ce{CS3^{2-}}[/latex]
- [latex]\ce{CS2}[/latex]
- [latex]\ce{CS}[/latex]
- predict the molecular shapes for [latex]\ce{CS3^{2-}}[/latex] and [latex]\ce{CS2}[/latex] and explain how you arrived at your predictions
- What is the molecular structure of the stable form of [latex]\ce{FNO2}[/latex]? ([latex]\ce{N}[/latex] is the central atom.)
- A compound with a molar mass of about 42 g/mol contains 85.7% carbon and 14.3% hydrogen. What is its molecular structure?
Show Selected Solutions
- The placement of the two sets of unpaired electrons in water forces the bonds to assume a tetrahedral arrangement, and the resulting [latex]\ce{HOH}[/latex] molecule is bent. The [latex]\ce{HBeH}[/latex] molecule (in which [latex]\ce{Be}[/latex] has only two electrons to bond with the two electrons from the hydrogens) must have the electron pairs as far from one another as possible and is therefore linear.
- Space must be provided for each pair of electrons whether they are in a bond or are present as lone pairs. Electron-pair geometry considers the placement of all electrons. Molecular structure considers only the bonding-pair geometry.
- As long as the polar bonds are compensated (for example. two identical atoms are found directly across the central atom from one another), the molecule can be non-polar.
- The answers are as follows:
- Number of valence electrons: [latex]\ce{S}[/latex] = 6, [latex]\ce{F}[/latex] = 7 each, total 48. A single line bond represents two electrons:
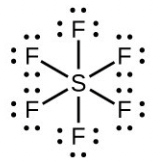 The total number of electrons used is 48; six bonds are formed and no non-bonded pairs exist. Therefore the molecule includes six regions of electron density and, from the table, the electron geometry is octahedral. Since no lone pairs exist, the electron geometry and molecular structure are the same.
The total number of electrons used is 48; six bonds are formed and no non-bonded pairs exist. Therefore the molecule includes six regions of electron density and, from the table, the electron geometry is octahedral. Since no lone pairs exist, the electron geometry and molecular structure are the same. - Number of valence electrons: [latex]\ce{P}[/latex] = 5, [latex]\ce{Cl}[/latex] = 7 each, total 40:
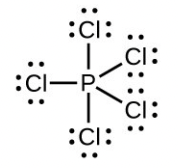
The total number of electrons is 40; there are five regions of electron density and, from the table, the geometry is trigonal bipyramid. Since no lone pairs exist on P, the electron geometry and molecular structure are the same. - Number of valence electrons: [latex]\ce{Be}[/latex] = 2, [latex]\ce{H}[/latex] = 1 each, total 4:
[latex]\ce{H:Be:H}[/latex]. There are only two regions of electron density and they must have a linear arrangement. These regions also correspond to the location of the bonds. Both the electron and molecular structures are linear. - Number of valence electrons: [latex]\ce{C}[/latex] = 4, [latex]\ce{H}[/latex] = 1 each, less one electron because of the positive charge, for a total of six electrons:
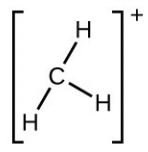 There are three regions of electron density coincident with the three bonds. Therefore the shape is trigonal planar for both the electron geometry and molecular structure.
There are three regions of electron density coincident with the three bonds. Therefore the shape is trigonal planar for both the electron geometry and molecular structure.
- Number of valence electrons: [latex]\ce{S}[/latex] = 6, [latex]\ce{F}[/latex] = 7 each, total 48. A single line bond represents two electrons:
-
Regions of High Electron Density Structure Formula Electrons Total Lone Pairs Electron Molecular [latex]\ce{ClF}[/latex] 42 6 1 octahedral square pyramidal [latex]\ce{ClO2^-}[/latex] 20 4 2 tetrahedral bent [latex]\ce{TeCl4^{2-}}[/latex] 36 6 2 octahedral square planar [latex]\ce{PCl3}[/latex] 26 4 1 tetrahedral trigonal pyramidal [latex]\ce{SeF4}[/latex] 34 5 1 trigonal bipyramidal seesaw [latex]\ce{PH2^-}[/latex] 8 4 2 tetrahedral bent (109°) -
Regions of High Electron Density Structure Formula Electrons Total Lone Pairs Electron Molecular [latex]\ce{CINO}[/latex] 18 3 1 trigonal planar bent (120°) [latex]\ce{CS2}[/latex] 16 2 0 linear linear [latex]\ce{Cl2CO}[/latex] 24 3 0 trigonal planar trigonal planar [latex]\ce{Cl2SO}[/latex] 26 4 1 tetrahedral trigonal pyramidal [latex]\ce{SO2F2}[/latex] 32 4 0 tetrahedral tetrahedral [latex]\ce{XeO2F2}[/latex] 34 5 1 trigonal bipyramidal seesaw [latex]\ce{ClOF2^+}[/latex] 26 4 1 tetrahedral trigonal pyramidal - All of these molecules and ions contain polar bonds. Only [latex]\ce{ClF5, CIO2^-, PCl3, SeF4, PH2^-}[/latex] and have dipole moments.
- The answers are as follows:
- [latex]\ce{CS2}[/latex] is linear and has no dipole moment.
- [latex]\ce{SeS2}[/latex] is bent. This leads to an overall dipole moment.
- The [latex]\ce{C–Cl}[/latex] and [latex]\ce{C–F}[/latex] bonds are not balanced—that is, the dipoles do not completely cancel. Therefore, it has a dipole moment.
- [latex]\ce{PCl3}[/latex] is trigonal pyramidal. Due to this shape, the dipoles of the bonds do not cancel and there is an overall dipole moment.
- The [latex]\ce{ClNO}[/latex] molecule is bent, leading to a dipole moment.
- P
- Non-polar
- The answers are as follows:
- tetrahedral;
- trigonal pyramidal;
- bent (109°);
- trigonal planar;
- bent (109°);
- bent (109°);
- [latex]\ce{CH3CCH}[/latex] tetrahedral, [latex]\ce{CH3CCH}[/latex] linear;
- tetrahedral,
- [latex]\ce{H2CCCH2}[/latex] linear; [latex]\ce{H2CCCH2}[/latex] trigonal planar

- The answers are as follows:
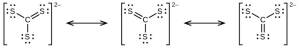
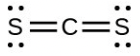
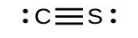
- [latex]\ce{CS3^{2-}}[/latex] includes three regions of electron density (all are bonds with no lone pairs); the shape is trigonal planar; [latex]\ce{CS2}[/latex] has only two regions of electron density (all bonds with no lone pairs); the shape is linear
- The empirical formula is [latex]\ce{CH2}[/latex] with a unit mass of l4. [latex]\frac{42}{14}[/latex] = 3 . Therefore, the Lewis structure is made from three units, but the atoms must be rearranged:
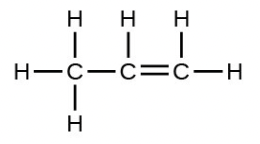
7.3 Molecular Polarity and Dipole Moments [Go to section 7.3]
- Describe the molecular geometry and hybridization of the [latex]\ce{N}[/latex], [latex]\ce{P}[/latex], or [latex]\ce{S}[/latex] atoms in each of the following compounds.
- [latex]\ce{H3PO4}[/latex] phosphoric acid, used in cola soft drinks
- [latex]\ce{NH4NO3}[/latex], ammonium nitrate, a fertilizer and explosive
- [latex]\ce{S2Cl2}[/latex], disulfur dichloride, used in vulcanizing rubber
- [latex]\ce{K4[O3POPO3]}[/latex], potassium pyrophosphate, an ingredient in some toothpastes
7.4 Valence Bond Theory [Go to section 7.4]
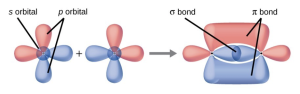
- Explain how σ and π bonds are similar and how they are different.
- Use valence bond theory to explain the bonding in [latex]\ce{F2, HF}, \text{and} \ce{ClBr}[/latex]. Sketch the overlap of the atomic orbitals involved in the bonds.
- Use valence bond theory to explain the bonding in [latex]\ce{O2}[/latex]. Sketch the overlap of the atomic orbitals involved in the bonds in [latex]\ce{O2}[/latex].
- How many σ and π bonds are present in the molecule [latex]\ce{HCN}[/latex]?
- A friend tells you [latex]\ce{N2}[/latex] has three π bonds due to overlap of the three p-orbitals on each [latex]\ce{N}[/latex] atom. Do you agree?
- Draw the Lewis structures for [latex]\ce{CO2}[/latex] and [latex]\ce{CO}[/latex], and predict the number of σ and π bonds for each molecule.
- [latex]\ce{CO2}[/latex]
- [latex]\ce{CO}[/latex]
- The bond energy of a [latex]\ce{C–C}[/latex] single bond averages 347 kJ mol-1; that of a [latex]\ce{C≡C}[/latex] triple bond averages 839 kJ mol-1. Explain why the triple bond is not three times as strong as a single bond.
- For the carbonate ion, [latex]\ce{CO3^2}[/latex], draw all of the resonance structures. Identify which orbitals overlap to create each bond.
Show Selected Solutions
- Similarities: Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons. Differences: σ bonds are stronger and result from end-to-end overlap and all single bonds are σ bonds; π bonds between the same two atoms are weaker because they result from side-by-side overlap, and multiple bonds contain one or more π bonds (in addition to a σ bond).
- Bonding: One σ bond and one π bond. The s orbitals are filled and do not overlap. The p orbitals overlap along the axis to form a σ bond and side by side to form the π bond.
- No, two of the p orbitals (one on each [latex]\ce{N}[/latex]) will be oriented end to end and will form a σ bond.
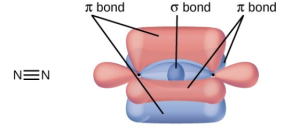
- A triple bond consists of one σ bond and two π bonds. A σ bond is stronger than a π bond due to greater overlap.
7.5 Hybrid Atomic Orbitals [Go to section 7.5]
- Why is the concept of hybridization required in valence bond theory?
- Give the shape that describes each hybrid orbital set:
- sp2
- sp3d
- sp
- sp3d2
- Explain why a carbon atom cannot form five bonds using sp[latex]^3[/latex]d hybrid orbitals.
- What is the hybridization of the central atom in each of the following?
- [latex]\ce{BeH2}[/latex]
- [latex]\ce{SF}[/latex]
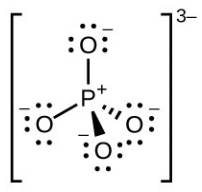
- [latex]\ce{PO4^{3-}}[/latex]
- [latex]\ce{PCl5}[/latex]
- A molecule with the formula AB3 could have one of four different shapes. Give the shape and the hybridization of the central A atom for each.
- Write Lewis structures for [latex]\ce{NF3}[/latex] and [latex]\ce{PF5}[/latex]. On the basis of hybrid orbitals, explain the fact that [latex]\ce{NF3, PF3}, \text{and } \ce{PF5}[/latex] are stable molecules, but [latex]\ce{NF5}[/latex] does not exist.
Show Selected Solutions
- Hybridization is introduced to explain the geometry of bonding orbitals in valance bond theory.
- There are no d orbitals in the valence shell of carbon.
- trigonal planar, sp2, trigonal pyramidal (one lone pair on A) sp3, T-shaped (two lone pairs on A sp3d, or (three lone pair on A) sp3d2
7.6 Multiple Bonds [Go to section 7.6]
- Sulfuric acid is manufactured by a series of reactions represented by the following equations:
[latex]\begin{array}{l} \ce{S8}(s) + 8\ce{O2}(g) \longrightarrow 8\ce{SO2}(g) \\ 2\ce{SO2}(g) + \ce{O2}(g) \longrightarrow 2\ce{SO3}(g) \\ \ce{SO3}(g) + \ce{H2O}(I) \longrightarrow \ce{H2SO4}(I) \\ \end{array}[/latex]
Draw a Lewis structure, predict the molecular geometry by VSEPR, and determine the hybridization of sulfur for the following:- circular [latex]\ce{S8}[/latex] molecule
- [latex]\ce{SO2}[/latex] molecule
- [latex]\ce{SO3}[/latex] molecule
- [latex]\ce{H2SO4}[/latex] molecule (the hydrogen atoms are bonded to oxygen atoms)
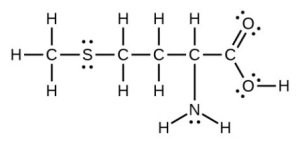
- Methionine, [latex]\ce{CH3SCH2CH2CH(NH2)COH}[/latex], is an amino acid found in proteins. Draw a Lewis structure of this compound. What is the hybridization type of each carbon, oxygen, the nitrogen, and the sulfur?
- Identify the hybridization of the central atom in each of the following molecules and ions that contain multiple bonds:
- [latex]\ce{ClNO}[/latex] ([latex]\ce{N}[/latex] is the central atom)
- [latex]\ce{CS2}[/latex]
- [latex]\ce{Cl2CO}[/latex] ([latex]\ce{C}[/latex] is the central atom)
- [latex]\ce{Cl2SO}[/latex] ([latex]\ce{S}[/latex] is the central atom)
- [latex]\ce{SO2F2}[/latex] ([latex]\ce{S}[/latex] is the central atom)
- [latex]\ce{XeO2F2}[/latex] ([latex]\ce{Xe}[/latex] is the central atom)
- [latex]\ce{ClOF2^+}[/latex] ([latex]\ce{Cl}[/latex] is the central atom)
- Two important industrial chemicals, ethene, [latex]\ce{C2H4}[/latex], and propene, [latex]\ce{C3H6}[/latex], are produced by the steam (or thermal) cracking process: [latex]\ce{2C3H8}(g) \longrightarrow \ce{C2H4}(g) + \ce{C3H6}(g) + \ce{CH4}(g) + \ce{H2}(g)[/latex] For each of the four carbon compounds, do the following:
- Draw a Lewis structure.
- Predict the geometry about the carbon atom.
- Determine the hybridization of each type of carbon atom.
- A useful solvent that will dissolve salts as well as organic compounds is the compound acetonitrile, [latex]\ce{H3CCN}[/latex]. It is present in paint strippers.
- Write the Lewis structure for acetonitrile, and indicate the direction of the dipole moment in the molecule.
- Identify the hybrid orbitals used by the carbon atoms in the molecule to form σ bonds.
- Describe the atomic orbitals that form the π bonds in the molecule. Note that it is not necessary to hybridize the nitrogen atom.
- Consider nitrous acid, [latex]\ce{HNO2} \ce{(HONO)}[/latex].
- Write a Lewis structure.
- What are the electron pair and molecular geometries of the internal oxygen and nitrogen atoms in the [latex]\ce{HNO2}[/latex] molecule?
- What is the hybridization on the internal oxygen and nitrogen atoms in [latex]\ce{HNO2}[/latex]?
- For each of the following molecules, indicate the hybridization requested and whether or not the electrons will be delocalized:
- ozone ([latex]\ce{O3}[/latex]) central [latex]\ce{O}[/latex] hybridization
- carbon dioxide ([latex]\ce{CO2}[/latex]) central [latex]\ce{C}[/latex] hybridization
- nitrogen dioxide ([latex]\ce{NO2}[/latex]) central [latex]\ce{N}[/latex] hybridization
- phosphate ion ([latex]\ce{PO4^{3-}}[/latex]) central [latex]\ce{P}[/latex] hybridization
- Identify the hybridization of each carbon atom in the following molecule. (The arrangement of atoms is given; you need to determine how many bonds connect each pair of atoms.)
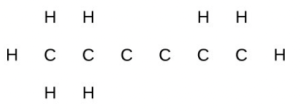
- Give the hybridization for the carbon atom in the [latex]\ce{HCN}[/latex] molecule.
- In addition to [latex]\ce{NF3}[/latex], two other fluoro derivatives of nitrogen are known: [latex]\ce{N2F4}[/latex] and [latex]\ce{N2F2}[/latex]. What shapes do you predict for these two molecules? What is the hybridization for the nitrogen in each molecule?
Show Selected Solutions
- The answers are as follows:
- [latex]\ce{S8}[/latex],each [latex]\ce{S}[/latex] has a bent (109°) geometry, sp2
- [latex]\ce{SO2}[/latex], bent (120°), sp3
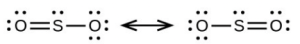
- [latex]\ce{SO3}[/latex], trigonal planar, sp2
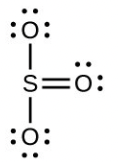
- [latex]\ce{H2SO4}[/latex], tetrahedral, sp3
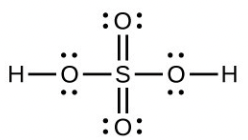
- [latex]\ce{S8}[/latex],each [latex]\ce{S}[/latex] has a bent (109°) geometry, sp2
- The answers are as follows:
- sp2
- sp
- sp2
- sp3
- sp3
- sp3d
- sp3
- The answers are as follows:
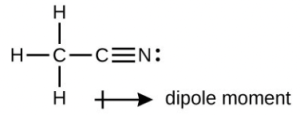
- The terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. The terminal carbon atom forms four σ bonds (three to the hydrogen atoms and one to the carbon) while the central carbon forms two σ bond (one to carbon and one to nitrogen).
- Each of the two πbonds is formed by overlap of a 2p orbital on carbon and a nitrogen 2p orbital.
- The answers are as follows:
- sp, delocalized
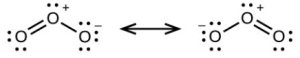
- sp, localized
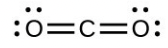
- sp2, delocalized

- sp3, delocalized
- sp, delocalized
- sp
7.7 Molecular Orbital Theory [Go to section 7.7]
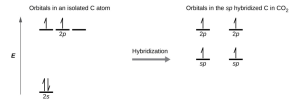
- Draw the orbital diagram for carbon in [latex]\ce{CO2}[/latex] showing how many carbon atom electrons are in each orbital.
- Sketch the distribution of electron density in the bonding and antibonding molecular orbitals formed from two s orbitals and from two p orbitals.
- Can a molecule with an odd number of electrons ever be diamagnetic? Explain why or why not.
- If molecular orbitals are created by combining five atomic orbitals from atom A and five atomic orbitals from atom B combine, how many molecular orbitals will result?
- Why are bonding molecular orbitals lower in energy than the parent atomic orbitals?
- Can a molecule with an even number of electrons ever be paramagnetic? Explain why or why not.
- Can a molecule with an even number of electrons ever be paramagnetic? Explain why or why not.
- Calculate the bond order for an ion with this configuration: [latex](\sigma_{2s})^2(\sigma_{2s}^*)^2(\sigma_{2pc}^2(\pi_{2py}\pi_{2pc})^4(\pi_{2py}^*\pi_{2pc}^*)^3[/latex]
- Determine the bond order of each member of the following groups, and determine which member of each group is predicted by the molecular orbital model to have the strongest bond.
- [latex]\ce{H2, H2^+, H2^-}[/latex]
- [latex]\ce{O2, O2^{2+}, O2^{2-}}[/latex]
- [latex]\ce{Li2, Be^+, Be2}[/latex]
- [latex]\ce{F2, F2^+, F2^-}[/latex]
- [latex]\ce{N2, N2^+, N2^-}[/latex]
- Predict the valence electron molecular orbital configurations for the following, and state whether they will be stable or unstable ions.
- [latex]\ce{Na2^{2+}}[/latex]
- [latex]\ce{Mg2^{2+}}[/latex]
- [latex]\ce{Al2^{2+}}[/latex]
- [latex]\ce{Si2^{2+}}[/latex]
- [latex]\ce{P2^{2}}[/latex]
- [latex]\ce{S2^{2+}}[/latex]
- [latex]\ce{F2^{2+}}[/latex]
- [latex]\ce{Ar2^{2+}}[/latex]
- Compare the atomic and molecular orbital diagrams to identify the member of each of the following pairs that has the highest first ionization energy (the most tightly bound electron) in the gas phase:
- [latex]\ce{H}[/latex] and [latex]\ce{H2}[/latex]
- [latex]\ce{N}[/latex] and [latex]\ce{N2}[/latex]
- [latex]\ce{O}[/latex] and [latex]\ce{O2}[/latex]
- [latex]\ce{C}[/latex] and [latex]\ce{C2}[/latex]
- [latex]\ce{B}[/latex] and [latex]\ce{B2}[/latex]
- For the first ionization energy for an [latex]\ce{N2}[/latex] molecule, what molecular orbital is the electron removed from?
- A friend tells you that the 2s orbital for fluorine starts off at a much lower energy than the 2s orbital for lithium, so the resulting σ2s molecular orbital in [latex]\ce{F2}[/latex] is more stable than in [latex]\ce{Li2}[/latex]. Do you agree?
- Which of the period 2 homonuclear diatomic molecules are predicted to be paramagnetic?
- What charge would be needed on [latex]\ce{F2}[/latex] to generate an ion with a bond order of 2?
- True or false: Boron contains 2s2 2p1 valence electrons, so only one p orbital is needed to form molecular orbitals.
- Explain why [latex]\ce{N2^{2+}}[/latex] is diamagnetic, while [latex]\ce{O2^4+}[/latex], which has the same number of valence electrons, is paramagnetic.
- Using the MO diagrams, predict the bond order for the stronger bond in each pair:
- [latex]\ce{B2}[/latex] or [latex]\ce{B2^+}[/latex]
- [latex]\ce{F2}[/latex] or [latex]\ce{F2^+}[/latex]
- [latex]\ce{O2}[/latex] or [latex]\ce{O2^{2+}}[/latex]
- [latex]\ce{C2^+}[/latex] or [latex]\ce{C2^-}[/latex]
Show Selected Solutions
 Each of the four electrons is in a separate orbital and overlaps with an electron on an oxygen atom.
Each of the four electrons is in a separate orbital and overlaps with an electron on an oxygen atom.- An odd number of electrons can never be paired, regardless of the arrangement of the molecular orbitals. It will always be paramagnetic.
- Bonding orbitals have electron density in close proximity to more than one nucleus. The interaction between the bonding positively charged nuclei and negatively charged electrons stabilizes the system.
- The pairing of the two bonding electrons lowers the energy of the system relative to the energy of the nonbonded electrons.
- The bond order is equal to half the difference between the number of bonding electrons and the number of antibonding electrons. The bond with the greatest bond order is predicted to be the strongest.
- [latex]\ce{H2}[/latex] bond order = 1, [latex]\ce{H2^+}[/latex] bond order = 0.5, [latex]\ce{H2^-}[/latex] bond order = 0.5, strongest bond is [latex]\ce{H2}[/latex];
- [latex]\ce{O2}[/latex] bond order = 2, [latex]\ce{O2^{2+}}[/latex] bond order = 3; [latex]\ce{O2^{2-}}[/latex] bond order = 1, strongest bond is [latex]\ce{O2^{2+}}[/latex];
- [latex]\ce{Li2}[/latex] bond order = 1, [latex]\ce{Be}[/latex] bond order = 0.5, [latex]\ce{Be2}[/latex] bond order = 0, strongest bond is [latex]\ce{Li2}[/latex];
- [latex]\ce{F2}[/latex] bond order = 1, bond order = 1.5, bond order = 0.5, strongest bond is ;
- [latex]\ce{N2}[/latex] bond order = 3, bond order = 2.5, bond order = 2.5, strongest bond is [latex]\ce{N2}[/latex].
- The answers are as follows:
- [latex]\ce{H2}[/latex]
- [latex]\ce{N2}[/latex]
- [latex]\ce{O}[/latex]
- [latex]\ce{C2}[/latex]
- [latex]\ce{B2}[/latex]
- Yes, fluorine is a smaller atom than [latex]\ce{Li}[/latex], so atoms in the 2s orbital are closer to the nucleus and more stable.
- 2+
- [latex]\ce{N2}[/latex] has s-p mixing, so the π orbitals are the last filled in [latex]\ce{N2^{2+}}[/latex] . [latex]\ce{O2}[/latex] does not have s-p mixing, so the σp orbital fills before the π orbital.

