Chapter 7: Advanced Theories of Covalent Bonding
7.3 Molecular Polarity and Dipole Moments
Learning Outcomes
- Assess the polarity of a molecule based on its bonding and structure
Molecular Polarity and Dipole Moment
As discussed previously, polar covalent bonds connect two atoms with differing electronegativities, leaving one atom with a partial positive charge ([latex]\delta[/latex]+) and the other atom with a partial negative charge ([latex]\delta[/latex]–), as the electrons are pulled toward the more electronegative atom. This separation of charge gives rise to a bond dipole moment. The magnitude of a bond dipole moment is represented by the Greek letter mu (µ) and is given by the formula shown below, where Q is the magnitude of the partial charges (determined by the electronegativity difference) and r is the distance between the charges:
[latex]\mu =\text{Qr}[/latex]
This bond moment can be represented as a vector, a quantity having both direction and magnitude (Figure 7.3.1). Dipole vectors are shown as arrows pointing along the bond from the less electronegative atom toward the more electronegative atom. A small plus sign is drawn on the less electronegative end to indicate the partially positive end of the bond. The length of the arrow is proportional to the magnitude of the electronegativity difference between the two atoms.

A whole molecule may also have a separation of charge, depending on its molecular structure and the polarity of each of its bonds. If such a charge separation exists, the molecule is said to be a polar molecule (or dipole); otherwise the molecule is said to be nonpolar. The dipole moment measures the extent of net charge separation in the molecule as a whole. We determine the dipole moment by adding the bond moments in three-dimensional space, taking into account the molecular structure.
For diatomic molecules, there is only one bond, so its bond dipole moment determines the molecular polarity. Homonuclear diatomic molecules such as [latex]\ce{Br2}[/latex] and [latex]\ce{N2}[/latex] have no difference in electronegativity, so their dipole moment is zero. For heteronuclear molecules such as [latex]\ce{CO}[/latex], there is a small dipole moment. For [latex]\ce{HF}[/latex], there is a larger dipole moment because there is a larger difference in electronegativity.
When a molecule contains more than one bond, the geometry must be taken into account. If the bonds in a molecule are arranged such that their bond moments cancel (vector sum equals zero), then the molecule is nonpolar. This is the situation in [latex]\ce{CO2}[/latex] (Figure 7.3.2). Each of the bonds is polar, but the molecule as a whole is nonpolar. From the Lewis structure, and using VSEPR theory, we determine that the [latex]\ce{CO2}[/latex] molecule is linear with polar [latex]\ce{C=O}[/latex] bonds on opposite sides of the carbon atom. The bond moments cancel because they are pointed in opposite directions. In the case of the water molecule, the Lewis structure again shows that there are two bonds to a central atom, and the electronegativity difference again shows that each of these bonds has a nonzero bond moment. In this case, however, the molecular structure is bent because of the lone pairs on [latex]\ce{O}[/latex], and the two bond moments do not cancel. Therefore, water does have a net dipole moment and is a polar molecule (dipole).

The [latex]\ce{OCS}[/latex] molecule has a structure similar to [latex]\ce{CO2}[/latex], but a sulfur atom has replaced one of the oxygen atoms. To determine if this molecule is polar, we draw the molecular structure. VSEPR theory predicts a linear molecule:
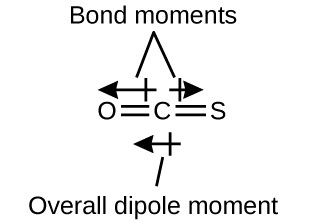
The [latex]\ce{C-O}[/latex] bond is considerably polar. Although [latex]\ce{C}[/latex] and [latex]\ce{S}[/latex] have the same electronegativity values as shown in Figure 7.3.3, [latex]\ce{S}[/latex] is slightly more electronegative than sulfur, and so the [latex]\ce{C-S}[/latex] bond is just slightly polar. The polarity of the [latex]\ce{C-O}[/latex] bond outweighs this slightly polar [latex]\ce{C-S}[/latex] bond. Because oxygen is more electronegative than sulfur, the oxygen end of the molecule is the negative end.

Chloromethane, [latex]\ce{CH3Cl}[/latex], is a tetrahedral molecule with three slightly polar [latex]\ce{C-H}[/latex] bonds and a more polar [latex]\ce{C-Cl}[/latex] bond. The relative electronegativities of the bonded atoms is [latex]\ce{H}<\ce{C}<\ce{Cl}[/latex], and so the bond moments all point toward the [latex]\ce{Cl}[/latex] end of the molecule and sum to yield a considerable dipole moment (the molecule is relatively polar).

For molecules of high symmetry such as [latex]\ce{BF3}[/latex] (trigonal planar), [latex]\ce{CH4}[/latex] (tetrahedral), [latex]\ce{PF5}[/latex] (trigonal bipyramidal), and [latex]\ce{SF6}[/latex] (octahedral), all the bonds are of identical polarity, and they are oriented in geometries that yield nonpolar molecules. However, molecules of less geometric symmetry may be polar even when all bond moments are identical. For these molecules, the directions of the equal bond moments are such that they sum to give a non-zero molecule dipole moment and a polar molecule. Examples of such molecules include H2S and NH3. A hydrogen atom is at the positive end and a nitrogen or sulfur atom is at the negative end of the polar bonds in these molecules:

To summarize, to be polar, a molecule must:
- Contain at least one polar covalent bond.
- Have a molecular structure such that the sum of the vectors of each bond dipole moment does not cancel.
Properties of Polar Molecules
Polar molecules tend to align when placed in an electric field with the positive end of the molecule oriented toward the negative plate and the negative end toward the positive plate (Figure 7.3.4). We can use an electrically charged object to attract polar molecules, but nonpolar molecules are not attracted. Also, polar solvents are better at dissolving polar substances, and nonpolar solvents are better at dissolving nonpolar substances.

Key Concepts and Summary
A dipole moment measures a separation of charge. For one bond, the bond dipole moment is determined by the difference in electronegativity between the two atoms. For a molecule, the overall dipole moment is determined by both the individual bond moments and how these dipoles are arranged in the molecular structure. Polar molecules (those with an appreciable dipole moment) interact with electric fields, whereas nonpolar molecules do not.
Try It
- Which of the following molecules have dipole moments?
- [latex]\ce{CS2}[/latex]
- [latex]\ce{SeS2}[/latex]
- [latex]\ce{CCl2F2}[/latex]
- [latex]\ce{PCl3}[/latex] ([latex]\ce{P}[/latex] is the central atom)
- [latex]\ce{ClNO}[/latex] ([latex]\ce{N}[/latex] is the central atom)
- A molecule with the formula AB2, in which A and B represent different atoms, could have one of three different shapes. Sketch and name the three different shapes that this molecule might have. Give an example of a molecule or ion for each shape.
Show Selected Solutions
- The answers are as follows:
- [latex]\ce{CS2}[/latex] is linear and has no dipole moment.
- [latex]\ce{SeS2}[/latex] is bent. This leads to an overall dipole moment.
- The [latex]\ce{C-Cl}[/latex] and [latex]\ce{C-F}[/latex] bonds are not balanced—that is, the dipoles do not completely cancel. Therefore, it has a dipole moment.
- [latex]\ce{PCl3}[/latex] is trigonal pyramidal. Due to this shape, the dipoles of the bonds do not cancel and there is an overall dipole moment.
- The [latex]\ce{ClNO}[/latex] molecule is bent, leading to a dipole moment.
- The three different possible shapes are

Glossary
bond dipole moment: separation of charge in a bond that depends on the difference in electronegativity and the bond distance represented by partial charges or a vector
dipole moment: property of a molecule that describes the separation of charge determined by the sum of the individual bond moments based on the molecular structure
vector: quantity having magnitude and direction
separation of charge in a bond that depends on the difference in electronegativity and the bond distance represented by partial charges or a vector
quantity having magnitude and direction
property of a molecule that describes the separation of charge determined by the sum of the individual bond moments based on the molecular structure

