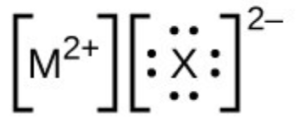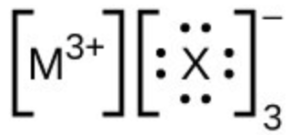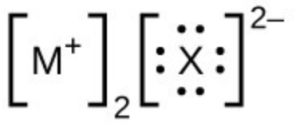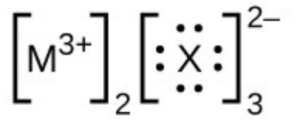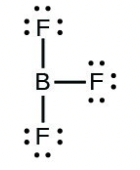Chapter 6: Chemical Bonding and Molecular Geometry
Chapter 6 Practice
6.1 Lewis Symbols and Structures [Go to section 6.1]
- Many monatomic ions are found in seawater, including the ions formed from the following list of elements. Write the Lewis symbols for the monatomic ions formed from the following elements:
- [latex]\ce{Ca}[/latex]
- [latex]\ce{K}[/latex]
- [latex]\ce{Br}[/latex]
- [latex]\ce{Sr}[/latex]
- [latex]\ce{F}[/latex]
- Write the Lewis symbols for each of the following ions:
- [latex]\ce{O^2-}[/latex]
- [latex]\ce{Ga^3+}[/latex]
- [latex]\ce{Li+}[/latex]
- [latex]\ce{N^3-}[/latex]
- In the Lewis structures listed below, M and X represent various elements in the third period of the periodic table. Write the formula of each compound using the chemical symbols of each element:
- Write the Lewis symbols of the ions in each of the following ionic compounds and the Lewis symbols of the atom from which they are formed:
- [latex]\ce{MgS}[/latex]
- [latex]\ce{Al2O3}[/latex]
- [latex]\ce{GaCl3}[/latex]
- [latex]\ce{K2O}[/latex]
- [latex]\ce{Li3N}[/latex]
- [latex]\ce{KF}[/latex]
- Write Lewis structures for the following:
- [latex]\ce{H2}[/latex]
- [latex]\ce{HBr}[/latex]
- [latex]\ce{PCl3}[/latex]
- [latex]\ce{SF2}[/latex]
- [latex]\ce{H2CCH2}[/latex]
- [latex]\ce{HNNH}[/latex]
- [latex]\ce{H2CNH}[/latex]
- [latex]\ce{NO-}[/latex]
- [latex]\ce{N2}[/latex]
- [latex]\ce{CO}[/latex]
- [latex]\ce{CN-}[/latex]
- Write the Lewis structure for the diatomic molecule [latex]\ce{P2}[/latex], an unstable form of phosphorus found in high-temperature phosphorus vapor.
- Write Lewis structures for the following:
- [latex]\ce{ClF3}[/latex]
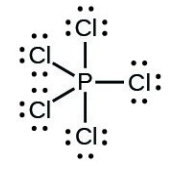
- [latex]\ce{PCl5}[/latex]
- [latex]\ce{BF3}[/latex]
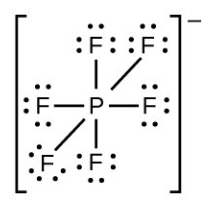
- [latex]\ce{PF6-}[/latex]
- Write Lewis structures for the following:
- [latex]\ce{O2}[/latex]
- [latex]\ce{H2CO}[/latex]
- [latex]\ce{AsF3}[/latex]
- [latex]\ce{ClNO}[/latex]
- [latex]\ce{SiCl4}[/latex]
- [latex]\ce{H3O+}[/latex]
- [latex]\ce{NH4+}[/latex]
- [latex]\ce{BF4-}[/latex]
- [latex]\ce{HCCH}[/latex]
- [latex]\ce{ClCN}[/latex]
- [latex]\ce{C2^2+}[/latex]
- Write Lewis structures for:
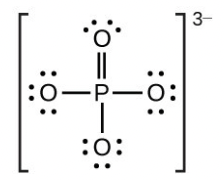
- [latex]\ce{PO4^3-}[/latex]
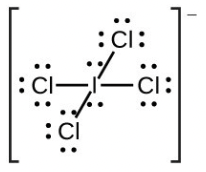
- [latex]\ce{IC4-}[/latex]
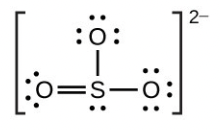
- [latex]\ce{SO3^2-}[/latex]
- [latex]\ce{HONO}[/latex]
- Write Lewis structures for the following:
- [latex]\ce{SeF6}[/latex]
- [latex]\ce{XeF4}[/latex]
- [latex]\ce{SeCl3+}[/latex]
- [latex]\ce{Cl2BBCl2}[/latex] (contains a [latex]\ce{B-B}[/latex] bond)
- Write Lewis structures for the following molecules or ions:
- [latex]\ce{SbH3}[/latex]
- [latex]\ce{XeF2}[/latex]
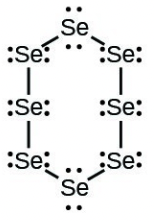
- [latex]\ce{Se8}[/latex] (a cyclic molecule with a ring of eight [latex]\ce{Se}[/latex] atoms)
- Correct the following statement: “The bonds in solid [latex]\ce{PbCl2}[/latex] are ionic; the bond in a [latex]\ce{HCl}[/latex] molecule is covalent. Thus, all of the valence electrons in [latex]\ce{PbCl2}[/latex] are located on the [latex]\ce{Cl-}[/latex] ions, and all of the valence electrons in a [latex]\ce{HCl}[/latex] molecule are shared between the [latex]\ce{H}[/latex] and [latex]\ce{Cl}[/latex] atoms.”
- Many planets in our solar system contain organic chemicals including methane ([latex]\ce{CH4}[/latex]) and traces of ethylene ([latex]\ce{C2H4}[/latex]), ethane ([latex]\ce{C2H6}[/latex]), propyne ([latex]\ce{H3CCCH}[/latex]), and diacetylene ([latex]\ce{HCCCCH}[/latex]). Write the Lewis structures for each of these molecules.
- Carbon tetrachloride was formerly used in fire extinguishers for electrical fires. It is no longer used for this purpose because of the formation of the toxic gas phosgene, [latex]\ce{Cl2CO}[/latex]. Write the Lewis structures for carbon tetrachloride and phosgene.
- How are single, double, and triple bonds similar? How do they differ?
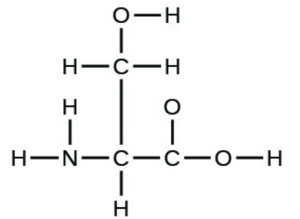
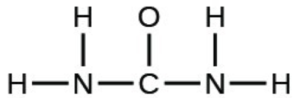
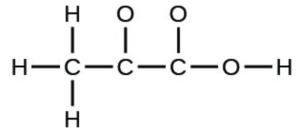
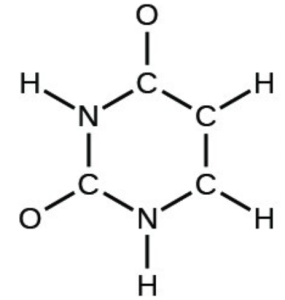
- The arrangement of atoms in several biologically important molecules is given below. Complete the Lewis structures of these molecules by adding multiple bonds and lone pairs. Do not add any more atoms.
- the amino acid serine:
- urea:
- pyruvic acid:
- uracil:
- carbonic acid:
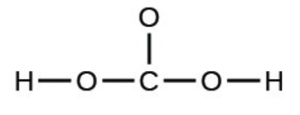
- the amino acid serine:
Show Selected Solutions
- The answers are as follows:
- [latex]\ce{Ca^2+}[/latex]
- [latex]\ce{K+}[/latex]
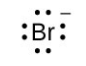
- [latex]\ce{Sr^2+}[/latex]
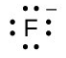
- The answers are as follows:
- [latex]\ce{MgS}[/latex]
- [latex]\ce{AlCl3}[/latex]
- [latex]\ce{Na2S}[/latex]
- [latex]\ce{Al2S3}[/latex]
- The answers are as follows:
- [latex]\ce{H–H}[/latex]
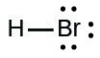
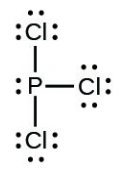
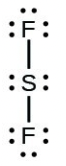
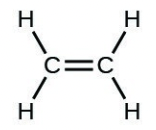
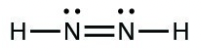
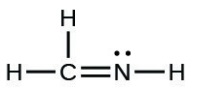
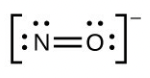
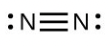
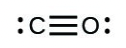
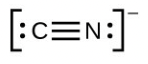
- The answers are as follows:
- The answers are as follows;
- The answers are as follows:
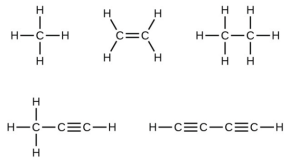
- Single bonds share a single pair of electrons, double bonds share two pairs, and triple bonds share three pairs. In a Lewis structure, a single bond is represented by a single straight line connecting the atoms in the bond. A double bond is represented by two lines and a triple bond is represented by three.
6.2 Electronegativity and Polarity [Go to section 6.2]
- From its position in the periodic table, determine which atom in each pair is more electronegative:
- [latex]\ce{Br}[/latex] or [latex]\ce{Cl}[/latex]
- [latex]\ce{N}[/latex] or [latex]\ce{O}[/latex]
- [latex]\ce{S}[/latex] or [latex]\ce{O}[/latex]
- [latex]\ce{P}[/latex] or [latex]\ce{S}[/latex]
- [latex]\ce{Si}[/latex] or [latex]\ce{N}[/latex]
- [latex]\ce{Ba}[/latex] or [latex]\ce{P}[/latex]
- [latex]\ce{N}[/latex] or [latex]\ce{K}[/latex]
- Explain the difference between a non-polar covalent bond, a polar covalent bond, and an ionic bond.
- From their positions in the periodic table, arrange the atoms in each of the following series in order of increasing electronegativity:
- [latex]\ce{C}[/latex], [latex]\ce{F}[/latex], [latex]\ce{H}[/latex], [latex]\ce{N}[/latex], [latex]\ce{O}[/latex]
- [latex]\ce{Br}[/latex], [latex]\ce{Cl}[/latex], [latex]\ce{F}[/latex], [latex]\ce{H}[/latex], [latex]\ce{I}[/latex]
- [latex]\ce{F}[/latex], [latex]\ce{H}[/latex], [latex]\ce{O}[/latex], [latex]\ce{P}[/latex], [latex]\ce{S}[/latex]
- [latex]\ce{Al}[/latex], [latex]\ce{H}[/latex], [latex]\ce{Na}[/latex], [latex]\ce{O}[/latex], [latex]\ce{P}[/latex]
- [latex]\ce{Ba}[/latex], [latex]\ce{H}[/latex], [latex]\ce{N}[/latex], [latex]\ce{O}[/latex], [latex]\ce{As}[/latex]
- From its position in the periodic table, determine which atom in each pair is more electronegative:
- [latex]\ce{Cl}[/latex] or [latex]\ce{S}[/latex]
- [latex]\ce{H}[/latex] or [latex]\ce{C}[/latex]
- [latex]\ce{Se}[/latex] or [latex]\ce{P}[/latex]
- [latex]\ce{C}[/latex] or [latex]\ce{Si}[/latex]
- Which atoms can bond to sulfur so as to produce a positive partial charge on the sulfur atom?
- From their positions in the periodic table, arrange the atoms in each of the following series in order of increasing electronegativity:
- [latex]\ce{Ca}[/latex], [latex]\ce{H}[/latex], [latex]\ce{K}[/latex], [latex]\ce{N}[/latex], [latex]\ce{Si}[/latex]
- [latex]\ce{Cl}[/latex], [latex]\ce{Cs}[/latex], [latex]\ce{Ge}[/latex], [latex]\ce{H}[/latex], [latex]\ce{Sr}[/latex]
- Identify the more polar bond in each of the following pairs of bonds:
- [latex]\ce{HF}[/latex] or [latex]\ce{HCl}[/latex]
- [latex]\ce{NO}[/latex] or [latex]\ce{CO}[/latex]
- [latex]\ce{SH}[/latex] or [latex]\ce{OH}[/latex]
- [latex]\ce{PCl}[/latex] or [latex]\ce{SCl}[/latex]
- [latex]\ce{CH}[/latex] or [latex]\ce{NH}[/latex]
- [latex]\ce{SO}[/latex] or [latex]\ce{PO}[/latex]
- [latex]\ce{CN}[/latex] or [latex]\ce{NN}[/latex]
- Which is the most polar bond?
- [latex]\ce{C-C}[/latex]
- [latex]\ce{C-H}[/latex]
- [latex]\ce{N-H}[/latex]
- [latex]\ce{O-H}[/latex]
- [latex]\ce{Se-H}[/latex]
- In the list below, identify the molecules that contain polar bonds.
- [latex]\ce{NH3}[/latex]
- [latex]\ce{CO}[/latex]
- [latex]\ce{CH4}[/latex]
- [latex]\ce{CSF2}[/latex]
Show Selected Solutions
- The answers are as follows:
- [latex]\ce{Cl}[/latex]
- [latex]\ce{O}[/latex]
- [latex]\ce{O}[/latex]
- [latex]\ce{S}[/latex]
- [latex]\ce{N}[/latex]
- [latex]\ce{P}[/latex]
- [latex]\ce{N}[/latex]
- The answers are as follows:
- [latex]\ce{H, C, N, O, F}[/latex]
- [latex]\ce{H, I, Br, Cl, F}[/latex]
- [latex]\ce{H, P, S, O, F}[/latex]
- [latex]\ce{Na, Al, H, P, O}[/latex]
- [latex]\ce{Ba, H, As, N, O}[/latex]
- [latex]\ce{N, O, F,} \text{ and }\ce{Cl}[/latex]
- The answers are as follows:
- [latex]\ce{HF}[/latex]
- [latex]\ce{CO}[/latex]
- [latex]\ce{OH}[/latex]
- [latex]\ce{PCl}[/latex]
- [latex]\ce{NH}[/latex]
- [latex]\ce{PO}[/latex]
- [latex]\ce{CN}[/latex]
- The answers are as follows:
- The [latex]\ce{N-H}[/latex] bond is polar.
- The [latex]\ce{C-O}[/latex] bond is polar.
- The [latex]\ce{C-H}[/latex] bond is non-polar
- The [latex]\ce{C-S}[/latex] bond is non-polar, but the [latex]\ce{C-F}[/latex] bond is polar.
6.3 Formal Charges and Resonance [Go to section 6.3]
- Write resonance forms that describe the distribution of electrons in each of the molecules or ions given below:
- sulfur dioxide, [latex]\ce{SO2}[/latex]
- carbonate ion, [latex]\ce{CO3^2-}[/latex]
- hydrogen carbonate ion, [latex]\ce{HCO3^-}[/latex] ([latex]\ce{C}[/latex] is bonded to an [latex]\ce{OH}[/latex] group and two [latex]\ce{O}[/latex] atoms)
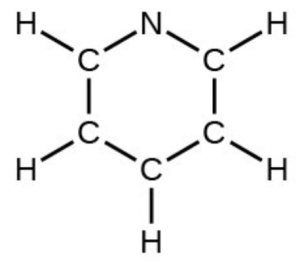
- pyridine:
- the allyl ion:
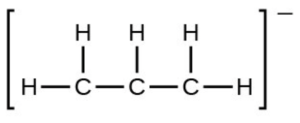
- Write the resonance forms of ozone, [latex]\ce{O3}[/latex], the component of the upper atmosphere that protects the Earth from ultraviolet radiation.
- Sodium nitrite, which has been used to preserve bacon and other meats, is an ionic compound. Write the resonance forms of the nitrite ion, [latex]\ce{NO2-}[/latex].
- In terms of the bonds present, explain why acetic acid, [latex]\ce{CH3CO2H}[/latex], contains two distinct types of carbon-oxygen bonds, whereas the acetate ion, formed by loss of a hydrogen ion from acetic acid, only contains one type of carbon-oxygen bond. The skeleton structures of these species are shown:
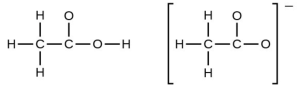
- Write the Lewis structures for the following, and include resonance structures where appropriate. Indicate which of the three has the strongest carbon-oxygen bond.
- [latex]\ce{CO2}[/latex]
- [latex]\ce{CO}[/latex]
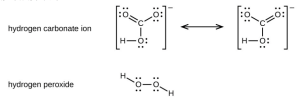
- Toothpastes containing sodium hydrogen carbonate (sodium bicarbonate) and hydrogen peroxide are widely used. Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule, with resonance forms where appropriate.
- Determine the formal charge of each element in the following:
- [latex]\ce{HCl}[/latex]
- [latex]\ce{CF4}[/latex]
- [latex]\ce{PCl3}[/latex]
- [latex]\ce{PF5}[/latex]
- Determine the formal charge of each element in the following:
- [latex]\ce{O2^2-}[/latex]
- [latex]\ce{H2O2}[/latex]
- Calculate the formal charge of chlorine in the molecules
- [latex]\ce{Cl2}[/latex]
- [latex]\ce{BeCl2}[/latex]
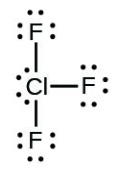
- [latex]\ce{ClF5}[/latex]
- Calculate the formal charge of each element in the following compounds and ions:
- [latex]\ce{F2CO}[/latex]
- [latex]\ce{NO-}[/latex]
- [latex]\ce{BF4-}[/latex]
- [latex]\ce{SnCl3-}[/latex]
- [latex]\ce{H2CCH2}[/latex]
- [latex]\ce{ClF3}[/latex]
- [latex]\ce{SeF6}[/latex]
- [latex]\ce{PO4^3-}[/latex]
- Draw all possible resonance structures for each of the compounds below. Determine the formal charge on each atom in each of the resonance structures:
- [latex]\ce{O3}[/latex]
- [latex]\ce{SO2}[/latex]
- [latex]\ce{NO2-}[/latex]
- [latex]\ce{NO3-}[/latex]
- Based on formal charge considerations, which of the following would likely be the correct arrangement of atoms in sulfur dioxide: [latex]\ce{OSO}[/latex] or [latex]\ce{SOO}[/latex]?
- Based on formal charge considerations, which of the following would likely be the correct arrangement of atoms in hypochlorous acid: [latex]\ce{HOCl}[/latex] or [latex]\ce{OClH}[/latex]?
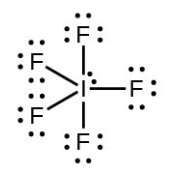
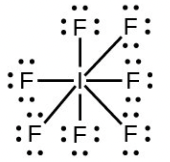
- Iodine forms a series of fluorides (listed below). Write Lewis structures for each of the four compounds and determine the formal charge of the iodine atom in each molecule:
- [latex]\ce{IF}[/latex]
- [latex]\ce{IF3}[/latex]
- [latex]\ce{IF5}[/latex]
- [latex]\ce{IF7}[/latex]
- Draw the structure of hydroxylamine, [latex]\ce{H3NO}[/latex], and assign formal charges; look up the structure. Is the actual structure consistent with the formal charges?
- Which of the following structures would we expect for nitrous acid? Determine the formal charges:
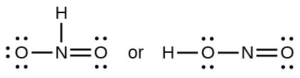
- Sulfuric acid is the industrial chemical produced in greatest quantity worldwide. About 90 billion pounds are produced each year in the United States alone. Write the Lewis structure for sulfuric acid, [latex]\ce{H2SO4}[/latex], which has two oxygen atoms and two [latex]\ce{OH}[/latex] groups bonded to the sulfur.
Show Selected Solutions

- The acetic acid molecule contains a [latex]\ce{C–O}[/latex] double bond and a [latex]\ce{C–O}[/latex] single bond. The acetate ion is described by two resonance structures that average the two [latex]\ce{C–O}[/latex] bonds.
-
Elements Bonding Electrons Non-bonded Electrons Valence Electrons Formal Charge (d) [latex]\ce{O}[/latex] 1 6 6 -1 (e) [latex]\ce{O}[/latex]
[latex]\ce{H}[/latex]2
14
06
10
0 -
Elements Bonding Electrons Non-bonded Electrons Valence Electrons Formal Charge (a) [latex]\ce{F}[/latex]
[latex]\ce{C}[/latex]
[latex]\ce{O}[/latex]1
4
26
0
47
4
60
0
0(b) [latex]\ce{N}[/latex]
[latex]\ce{O}[/latex]2
24
45
6-1
0(c) [latex]\ce{B}[/latex]
[latex]\ce{F}[/latex]4
10
63
7-1
0(d) [latex]\ce{Sn}[/latex]
[latex]\ce{Cl}[/latex]3
12
64
7-1
0(e) [latex]\ce{C}[/latex]
[latex]\ce{H}[/latex]4
10
04
10
0(f) [latex]\ce{Cl}[/latex]
[latex]\ce{F}[/latex]3
14
67
70
0(g) [latex]\ce{Se}[/latex]
[latex]\ce{F}[/latex]6
10
66
70
0(h) [latex]\ce{P}[/latex]
[latex]\ce{O}[/latex]4
10
65
6+1
-1 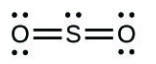
- The answers are as follows:
- Formal Charge 0.
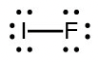
- Formal Charge 0.
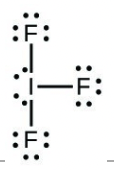
- Formal Charge 0.
- Formal Charge 0.
- Formal Charge 0.
- The second form, having all zero formal charges, is more likely:

6.4 Strengths of Ionic and Covalent Bonds [Go to section 6.4]
- Using the bond energies in Table 1, determine the approximate enthalpy change for each of the following reactions:
- [latex]{\ce{Cl}}_{2}\left(g\right)+3{\ce{F}}_{2}\left(g\right)\rightarrow 2{\ce{ClF}}_{3}\left(g\right)[/latex]
- [latex]{\ce{H}}_{2}\ce{C}={\ce{CH}}_{2}\left(g\right)+{\ce{H}}_{2}\left(g\right)\rightarrow{\ce{H}}_{3}{\ce{CCH}}_{3}\left(g\right)[/latex]
- [latex]2{\ce{C}}_{2}{\ce{H}}_{6}\left(g\right)+7{\ce{O}}_{2}\left(g\right)\rightarrow 4{\ce{CO}}_{2}\left(g\right)+6{\ce{H}}_{2}\ce{O}\left(g\right)[/latex]
- Using the bond energies in Table 1, determine the approximate enthalpy change for each of the following reactions:
- [latex]{\ce{H}}_{2}\left(g\right)+{\ce{Br}}_{2}\left(g\right)\rightarrow 2\ce{HBr}\left(g\right)[/latex]
- [latex]{\ce{CH}}_{4}\left(g\right)+{\ce{I}}_{2}\left(g\right)\rightarrow{\ce{CH}}_{3}\ce{I}\left(g\right)+\ce{HI}\left(g\right)[/latex]
- [latex]{\ce{C}}_{2}{\ce{H}}_{4}\left(g\right)+3{\ce{O}}_{2}\left(g\right)\rightarrow 2{\ce{CO}}_{2}\left(g\right)+2{\ce{H}}_{2}\ce{O}\left(g\right)[/latex]
- Using the standard enthalpy of formation data in Standard Thermodynamic Properties for Selected Substances, calculate the bond energy of the carbon-sulfur double bond in [latex]\ce{CS2}[/latex].
- When a molecule can form two different structures, the structure with the stronger bonds is usually the more stable form. Use bond energies to predict the correct structure of the hydroxylamine molecule:
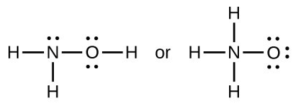
- Using the standard enthalpy of formation data in Standard Thermodynamic Properties for Selected Substances, determine which bond is stronger: the [latex]\ce{P-Cl}[/latex] bond in [latex]\ce{PCl3}[/latex](g) or in [latex]\ce{PCl5}[/latex](g)?
- Complete the following Lewis structure by adding bonds (not atoms), and then indicate the longest bond:

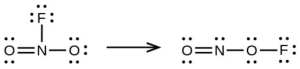
- Use the bond energy to calculate an approximate value of ΔH for the following reaction. Which is the more stable form of [latex]\ce{FNO2}[/latex]?
- For which of the following substances is the least energy required to convert one mole of the solid into separate ions?
- [latex]\ce{MgO}[/latex]
- [latex]\ce{SrO}[/latex]
- [latex]\ce{KF}[/latex]
- [latex]\ce{CsF}[/latex]
- [latex]\ce{MgF2}[/latex]
- The lattice energy of [latex]\ce{LiF}[/latex] is 1023 kJ/mol, and the [latex]\ce{Li-F}[/latex] distance is 200.8 pm. [latex]\ce{NaF}[/latex] crystallizes in the same structure as [latex]\ce{LiF}[/latex] but with a [latex]\ce{Na-F}[/latex] distance of 231 pm. Which of the following values most closely approximates the lattice energy of [latex]\ce{NaF}[/latex]: 510, 890, 1023, 1175, or 4090 kJ/mol? Explain your choice.
- The lattice energy of [latex]\ce{LiF}[/latex] is 1023 kJ/mol, and the [latex]\ce{Li-F}[/latex] distance is 201 pm. [latex]\ce{MgO}[/latex] crystallizes in the same structure as [latex]\ce{LiF}[/latex] but with a [latex]\ce{Mg-O}[/latex] distance of 205 pm. Which of the following values most closely approximates the lattice energy of [latex]\ce{MgO}[/latex]: 256 kJ/mol, 512 kJ/mol, 1023 kJ/mol, 2046 kJ/mol, or 4090 kJ/mol? Explain your choice.
- The reaction of a metal, [latex]\ce{M}[/latex], with a halogen, [latex]\ce{X2}[/latex], proceeds by an exothermic reaction as indicated by this equation: [latex]\ce{M}(s)+\ce{X2}(g)\longrightarrow\ce{MX2}(s).[/latex] For each of the following, indicate which option will make the reaction more exothermic. Explain your answers.
- a large radius vs. a small radius for [latex]\ce{M^+2}[/latex]
- a high ionization energy vs. a low ionization energy for [latex]\ce{M}[/latex]
- an increasing bond energy for the halogen
- a decreasing electron affinity for the halogen
- an increasing size of the anion formed by the halogen
- Which compound in each of the following pairs has the larger lattice energy? Note: [latex]\ce{Ba^2+}[/latex] and [latex]\ce{K+}[/latex] have similar radii; [latex]\ce{S^2-}[/latex] and [latex]\ce{Cl-}[/latex] have similar radii. Explain your choices.
- [latex]\ce{K2O}[/latex] or [latex]\ce{Na2O}[/latex]
- [latex]\ce{K2S}[/latex] or [latex]\ce{BaS}[/latex]
- [latex]\ce{KCl}[/latex] or [latex]\ce{BaS}[/latex]
- [latex]\ce{BaS}[/latex] or [latex]\ce{BaCl2}[/latex]
- Which compound in each of the following pairs has the larger lattice energy? Note: [latex]\ce{Mg^2+}[/latex] and [latex]\ce{Li+}[/latex] have similar radii; [latex]\ce{O^2-}[/latex] and [latex]\ce{F-}[/latex] have similar radii. Explain your choices.
- [latex]\ce{MgO}[/latex] or [latex]\ce{MgSe}[/latex]
- [latex]\ce{LiF}[/latex] or [latex]\ce{MgO}[/latex]
- [latex]\ce{Li2O}[/latex] or [latex]\ce{LiCl}[/latex]
- [latex]\ce{Li2Se}[/latex] or [latex]\ce{MgO}[/latex]
- Which of the following compounds requires the most energy to convert one mole of the solid into separate ions?
- [latex]\ce{K2S}[/latex]
- [latex]\ce{K2O}[/latex]
- [latex]\ce{CaS}[/latex]
- [latex]\ce{Cs2S}[/latex]
- [latex]\ce{CaO}[/latex]
- Which of the following compounds requires the most energy to convert one mole of the solid into separate ions?
- [latex]\ce{MgO}[/latex]
- [latex]\ce{SrO}[/latex]
- [latex]\ce{KF}[/latex]
- [latex]\ce{CsF}[/latex]
- [latex]\ce{MgF2}[/latex]
- The lattice energy of [latex]\ce{KF}[/latex] is 794 kJ/mol, and the inter-ionic distance is 269 pm. The [latex]\ce{Na-F}[/latex] distance in [latex]\ce{NaF}[/latex], which has the same structure as [latex]\ce{KF}[/latex], is 231 pm. Which of the following values is the closest approximation of the lattice energy of [latex]\ce{NaF}[/latex]: 682 kJ/mol, 794 kJ/mol, 924 kJ/mol, 1588 kJ/mol, or 3175 kJ/mol? Explain your answer.
Show Selected Solutions
- The answers are as follows:
- [latex]\begin{array}{lcl} \Delta H° & = & \sum \Delta_{\text{bonds broken}} - \sum \Delta_{\text{bonds formed}} \\ & = & 2\Delta_{\ce{Cl-Cl}} + 3\Delta_{\ce{F-F}} - 6\Delta_{\ce{Cl-F}} \\ & = & -564 \text{ kJ} \\ \end{array}[/latex]
- [latex]\begin{array}{lcl} \Delta H° & = & \sum \Delta_{\text{bonds broken}} - \sum \Delta_{\text{bonds formed}} \\ & = & \Delta_{\ce{C-C}} + 4\Delta_{\ce{C-H}} + \Delta_{\ce{H-H}} - \Delta_{\ce{C-C}} - 6\Delta_{\ce{C-H}} \\ & = & 611 + 4(415) + 436 - 345 - 6(415) \\ & = & -128 \text{ kJ} \\ \end{array}[/latex]
- [latex]\begin{array}{lcl} \Delta H° & = & \sum \Delta_{\text{bonds broken}} - \sum \Delta_{\text{bonds formed}} \\ & = & 2\Delta_{\ce{C-C}} + 12\Delta_{\ce{C-H}} + 7\Delta_{\ce{O-O}} - 8\Delta_{\ce{C-O}} - 12\Delta_{\ce{O-H}} \\ & = & 2(345) + 12(415) + 7(496) - 8(741) - 12(464) \\ & = & -2354 \text{ kJ} \\ \end{array}[/latex]
- [latex]\begin{array}{lr} \ce{CS2}(g) \longrightarrow \ce{C}\text{(graphite) } + \ce{2S}(s) & \Delta H^°_1 = \Delta H^°_{\text{f[}\ce{CS2} (g)]} \\ \ce{C}\text{(graphite)} \longrightarrow \ce{C}(g) & \Delta H^°_2 = \Delta H^°_{\text{f}\ce{C}(g)} \\ 2\ce{S}(s) \longrightarrow 2\ce{S}(g) & 2\Delta H^°_3 = 2\Delta H^°_{\text{f}\ce{S}(g)} \\ \end{array}[/latex]
[latex]\begin{array}{lll} \Delta_{\ce{CS2}} & = \Delta H^° = -\Delta H^°_{\text{f[}\ce{CS2}(g)]} + \Delta H^°_{\text{f}\ce{C}(g)} + 2\Delta H^°_{\text{f}\ce{S}(g)} \\ & = -116.9 + 716.681 + 2(278.81) \\ & = 1157.4 \text{ kJ mol}^{-1} \\ \Delta_{\ce{C=S}} & = \frac{1157.4}{2} = 578.7 \text{ kJ mol}^{-1} \text{ of C = S bonds} \\ \end{array}[/latex] - [latex]\begin{array}{lr} \ce{PCl3}(g) \longrightarrow \frac{1}{4}\ce{P4}(s) + \frac{3}{2} \ce{Cl}(s) & \Delta H^°_1 = -\Delta H^°_{\text{f[}\ce{PCl3} (g)]} \\ \frac{1}{4} \ce{P4}(s) \longrightarrow \ce{P}(g) & \Delta H^°_2 = \Delta H^°_{\text{f}\ce{P}(g)} \\ \frac{3}{2}\ce{Cl2}(g) \longrightarrow 3\ce{Cl}(g) & 3\Delta H^°_3 = 3\Delta H^°_{\text{f}\ce{Cl}(g)} \\ \end{array}[/latex]
[latex]\begin{array}{lll} \Delta_{\ce{PCl3}} & = \Delta H^° = -\Delta H^°_{\text{f[}\ce{PCl3}(g)]} + \Delta H^°_{\text{f}\ce{P}(g)} + 2\Delta H^°_{\text{f}\ce{Cl}(g)} \\ & = 287.0 + 314.64 + 3(121.3) = 965.54 \text{kJ mol}^{-1} \\ \Delta_{\ce{C=S}} & = \frac{965.54 \text{kJ}}{3} = 321.8 \text{ kJ mol}^{-1} \text{ of bonds} \\ \end{array}[/latex]Proceeding in the same manners, [latex]-\Delta H_{\text{f}[\ce{PCl5}, (g)]}[/latex] = 374.9 kJ mol–1. The P(g) and the 5Cl(g) contribute 921.14 kJ; then [latex]\Delta \text{F}_{\ce{PCI5}}[/latex] = 1296.04 kJ and [latex]\Delta_{\ce{P-Cl}} = \frac{1296.04 \text{ kJ/mol}}{5}[/latex] = 259.2 kJ/mol of bonds. The P–Cl bond in PCl3 is stronger. - the left hand arrangement: [latex]\ce{O = N}[/latex] not listed, [latex]\ce{N–F}[/latex] 270, [latex]\ce{N–O}[/latex] 200; the right hand arrangement: [latex]\ce{O = N}[/latex] not listed, [latex]\ce{N–O}[/latex] 200, [latex]\ce{O–F}[/latex] 185; the bond energy of [latex]\ce{O = N}[/latex] does not matter because it must be the same in both cases, the form on the right has a bond energy of X + 470; that on the right, X +385; the form on the left is more stable.
- The lattice energy is given by, [latex]U = C(\frac{Z^+Z^-}{R_o})[/latex] where Ro is the interatomic distance. The charges are the same in both [latex]\ce{LiF} \text{ and } \ce{NaF}[/latex]. The major difference is expected to be the interatomic distance 2.008 Å versus 2.31 Å. From the data for [latex]\ce{LiF}[/latex], with Z+Z- = –1, [latex]C = \frac{UR_o}{Z^+Z^-} = \frac{1023 × 2.008}{-1} = -2054 \text{ kJ Å mol}^{-1}[/latex]. Then, [latex]U_{\ce{NaF}} = \frac{-2054 \text{ kJ Å } mol^{-1} (-1)}{2.31 Å} = 889 \text{kJ mol}^{-1} \text{ or } 890 \text{kJ mol}^{-1}[/latex]
- In each case, think about how it would affect the Born-Haber cycle. Recall that the more negative the overall value, the more exothermic the reaction is. (a) The smaller the radius of the cation, the shorter the inter-ionic distance and the greater the lattice energy would be. Since the lattice energy is negative in the Born-Haber cycle, this would lead to a more exothermic reaction. (b) A lower ionization energy is a lower positive energy in the Born-Haber cycle. This would make the reaction more exothermic, as a smaller positive value is “more exothermic.” (c) As in part (b), the bond energy is a positive energy. The lower it is, the more exothermic the reaction will be. (d) A higher electron affinity is more negative. In the Born-Haber cycle, the more negative the electron affinity, the more exothermic the overall reaction. (e) The smaller the radius of the anion, the shorter the inter-ionic distance and the greater the lattice energy would be. Since the lattice energy is negative in the Born-Haber cycle, this would lead to a more exothermic reaction.
- The answers are as follows
- [latex]\ce{MgO}[/latex]; selenium has larger radius than oxygen and, therefore, a larger inter-ionic distance and thus, a larger smaller lattice energy than [latex]\ce{MgO}[/latex];
- [latex]\ce{MgO}[/latex]; the higher charges on [latex]\ce{Mg}[/latex] and [latex]\ce{O}[/latex], given the similar radii of the ions, leads to a larger lattice energy;
- [latex]\ce{Li2O}[/latex]; the higher charge on [latex]\ce{O^{2-}}[/latex] leads to a larger energy; additionally, [latex]\ce{Cl^-}[/latex] is larger than [latex]\ce{O^{2-}}[/latex]; this leads to a larger inter-ionic distance in [latex]\ce{LiCl}[/latex] and a lower lattice energy;
- [latex]\ce{MgO}[/latex]; the higher charge on [latex]\ce{Mg}[/latex] leads to a larger lattice energy
- [latex]\ce{MgO}[/latex]