Chapter 8: Stoichiometry of Chemical Reactions
Chapter 8 Practice
8.1 Chemical Equations and Stoichiometric Relationships [Go to section 8.1]
- Determine the number of moles and the mass requested for each reaction in Exercise 44.
- Write the balanced equation, then outline the steps necessary to determine the information requested in each of the following: (Ch 8 Problem 3)
- The number of moles and the mass of [latex]\ce{Mg}[/latex] required to react with 5.00 g of [latex]\ce{HCl}[/latex] and produce [latex]\ce{MgCl2}[/latex] and [latex]\ce{H2}[/latex].
- The number of moles and the mass of oxygen formed by the decomposition of 1.252 g of silver(I) oxide.
- The number of moles and the mass of magnesium carbonate, [latex]\ce{MgCO3}[/latex], required to produce 283 g of carbon dioxide. ([latex]\ce{MgO}[/latex] is the other product.)
- The number of moles and the mass of water formed by the combustion of 20.0 kg of acetylene, [latex]\ce{C2H2}[/latex], in an excess of oxygen.
- The number of moles and the mass of barium peroxide, [latex]\ce{BaO2}[/latex], needed to produce 2.500 kg of barium oxide, [latex]\ce{BaO}[/latex] ([latex]\ce{O2}[/latex] is the other product.)
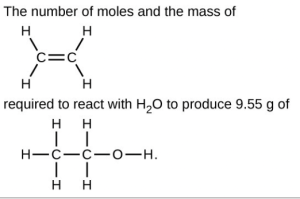
- Carborundum is silicon carbide, [latex]\ce{SiC}[/latex], a very hard material used as an abrasive on sandpaper and in other applications. It is prepared by the reaction of pure sand, [latex]\ce{SiO2}[/latex], with carbon at high temperature. Carbon monoxide, [latex]\ce{CO}[/latex], is the other product of this reaction. Write the balanced equation for the reaction and calculate how much [latex]\ce{SiO2}[/latex] is required to produce 3.00 kg of [latex]\ce{SiC}[/latex].
- [latex]\ce{H2}[/latex] is produced by the reaction of 118.5 mL of a 0.8775-M solution of [latex]\ce{H3PO4}[/latex] according to the following equation: [latex]\ce{2Cr}+\ce{2H3PO4}\rightarrow \ce{3H2}+\ce{2CrPO4}[/latex]. Determine the number of moles and mass of [latex]\ce{H2}[/latex].
- A compact car gets 37.5 miles per gallon on the highway. If gasoline contains 84.2% carbon by mass and has a density of 0.8205 g/mL, determine the mass of carbon dioxide produced during a 500-mile trip (3.785 liters per gallon).
- [latex]\ce{I2}[/latex] is produced by the reaction of 0.4235 mol of [latex]\ce{CuCl2}[/latex] according to the following equation: [latex]\ce{2CuCl2}+{4KI}\longrightarrow \ce{2CuI}+\ce{4KCl}+\ce{I2}[/latex].
- How many molecules of [latex]\ce{I2}[/latex] are produced
- What mass of [latex]\ce{I2}[/latex] is produced?
- What volume of a 0.2089 M [latex]\ce{KI}[/latex] solution contains enough [latex]\ce{KI}[/latex] to react exactly with the [latex]\ce{Cu(NO3)2}[/latex] in 43.88 mL of a 0.3842 M solution of [latex]\ce{Cu(NO3)2}[/latex]? [latex]\ce{2Cu(NO3)2}+\ce{4KI}\longrightarrow \ce{2CuI}+\ce{I2}+\ce{4KNO3}[/latex]
- What mass of silver oxide, [latex]\ce{Ag2O}[/latex], is required to produce 25.0 g of silver sulfadiazine, [latex]\ce{AgC_{10}H9N4SO2}[/latex], from the reaction of silver oxide and sulfadiazine? [latex]\ce{2C_{10}H_{10}N4SO2}+\ce{Ag2O}\longrightarrow \ce{2AgC_{10}H9N4SO2}+\ce{H2O}[/latex]
- The toxic pigment called white lead, [latex]\ce{Pb3(OH)2(CO3)2}[/latex], has been replaced in white paints by rutile, [latex]\ce{TiO2}[/latex]. How much rutile (g) can be prepared from 379 g of an ore that contains 88.3% ilmenite ([latex]\ce{FeTiO3}[/latex]) by mass?[latex]\ce{2FeTiO3}+\ce{4HCl}+\ce{Cl2}\longrightarrow \ce{2FeCl3}+\ce{2TiO2}+\ce{2H2O}[/latex]
- Automotive air bags inflate when a sample of sodium azide, [latex]\ce{NaN3}[/latex], is very rapidly decomposed.[latex]\ce{2NaN3}(s)\longrightarrow \ce{2Na}(s)+\ce{3N2}(g)[/latex] What mass of sodium azide is required to produce 2.6 ft3 (73.6 L) of nitrogen gas with a density of 1.25 g/L?
- What mass of sodium bicarbonate [latex]\ce{NaHCO3}[/latex] is required to completely neutralize 1.0 kg of spilled sulfuric acid ([latex]\ce{H2SO4}[/latex])?
- What volume of a 0.750 M solution of hydrochloric acid, a solution of [latex]\ce{HCl}[/latex], can be prepared from the [latex]\ce{HCl}[/latex] produced by the reaction of 25.0 g of [latex]\ce{NaCl}[/latex] with an excess of sulfuric acid? [latex]\ce{NaCl}(s)+\ce{H2SO4}(l)\longrightarrow\ce{HCl}(g)+\ce{NaHSO4}(s)[/latex]
Show Selected Solutions
- The answers are as follows:
- 0.0686 mol, 1.67 g
- 2.701 × 10-3 mol, 0.08644 g
- 6.43 mol, 542 g
- 713 mol, 12.8 kg
- 16.31 mol, 2762 g
- 0.207 mol, 5.81 g
- [latex]\ce{SiO2} + 3\ce{C} \longrightarrow \ce{SiC} + 2\ce{CO} , 4.5 \text{ kg } \ce{SiO2}[/latex]
- 1.28 × 105 g [latex]\ce{CO2}[/latex]
- 161.4 mL [latex]\ce{KI}[/latex] solution
- 176 g [latex]\ce{TiO2}[/latex]
- 1.7 kg
8.2 Precipitation Reactions and Solubility [Go to section 8.2] & 8.3 Other Types of Chemical Reactions [Go to section 8.3]
- Indicate what type, or types, of reaction each of the following represents:
- [latex]\text{Ca(}s\text{)}+{\text{Br}}_{2}\text{(}l\text{)}\rightarrow{\text{CaBr}}_{2}\text{(}s\text{)}[/latex]
- [latex]\text{Ca}{\text{(OH)}}_{2}\text{(}aq\text{)}+2\text{HBr(}aq\text{)}\rightarrow{\text{CaBr}}_{2}\text{(}aq\text{)}+2{\text{H}}_{2}\text{O(}l\text{)}[/latex]
- [latex]{\text{C}}_{6}{\text{H}}_{12}\text{(}l\text{)}+9{\text{O}}_{2}\text{(}g\text{)}\rightarrow 6{\text{CO}}_{2}\text{(}g\text{)}+6{\text{H}}_{2}\text{O(}g\text{)}[/latex]
- Indicate what type, or types, of reaction each of the following represents:
- [latex]{\text{H}}_{2}\text{O(}g\text{)}+\text{C(}s\text{)}\rightarrow\text{CO(}g\text{)}+{\text{H}}_{2}\text{(}g\text{)}[/latex]
- [latex]{\text{2KClO}}_{3}\text{(}s\text{)}\rightarrow 2\text{KCl(}s\text{)}+3{\text{O}}_{2}\text{(}g\text{)}[/latex]
- [latex]\text{Al}{\text{(OH)}}_{3}\text{(}aq\text{)}+3\text{HCl(}aq\text{)}\rightarrow{\text{AlBr}}_{3}\text{(}aq\text{)}+3{\text{H}}_{2}\text{O}\text{(}l\text{)}[/latex]
- [latex]\text{Pb}{\text{(}{\text{NO}}_{3}\text{)}}_{2}\text{(}aq\text{)}+{\text{H}}_{2}{\text{SO}}_{4}\text{(}aq\text{)}\rightarrow{\text{PbSO}}_{4}\text{(}s\text{)}+2{\text{HNO}}_{3}\text{(}aq\text{)}[/latex]
- Silver can be separated from gold because silver dissolves in nitric acid while gold does not. Is the dissolution of silver in nitric acid an acid-base reaction or an oxidation-reduction reaction? Explain your answer.
- Determine the oxidation states of the elements in the following compounds:
- [latex]\ce{NaI}[/latex]
- [latex]\ce{GdCl}[/latex]
- [latex]\ce{LiNO3}[/latex]
- [latex]\ce{H2Se}[/latex]
- [latex]\ce{Mg2Si}[/latex]
- [latex]\ce{RbO2}[/latex], rubidium superoxide
- [latex]\ce{HF}[/latex]
- Determine the oxidation states of the elements in the compounds listed. None of the oxygen-containing compounds are peroxides or superoxides.
- [latex]\ce{H3PO4}[/latex]
- [latex]\ce{Al(OH)3}[/latex]
- [latex]\ce{SeO2}[/latex]
- [latex]\ce{lKNO2}[/latex]
- [latex]\ce{In2S3}[/latex]
- [latex]\ce{P4O6}[/latex]
- Determine the oxidation states of the elements in the compounds listed. None of the oxygen-containing compounds are peroxides or superoxides.
- [latex]\ce{H2SO4}[/latex]
- [latex]\ce{Ca(OH)2}[/latex]
- [latex]\ce{BrOH}[/latex]
- [latex]\ce{ClNO2}[/latex]
- [latex]\ce{TiCl4}[/latex]
- [latex]\ce{NaH}[/latex]
- Classify the following as acid-base reactions or oxidation-reduction reactions:
- [latex]{\text{Na}}_{2}\text{S(}aq\text{)}+2\text{HCl(}aq\text{)}\rightarrow 2\text{NaCl(}aq\text{)}+{\text{H}}_{2}\text{S(}g\text{)}[/latex]
- [latex]2\text{Na(}s\text{)}+2\text{HCl(}aq\text{)}\rightarrow 2\text{NaCl(}aq\text{)}+{\text{H}}_{2}\text{(}g\text{)}[/latex]
- [latex]\text{Mg(}s\text{)}+{\text{Cl}}_{2}\text{(}g\text{)}\rightarrow{\text{MgCl}}_{2}\text{(}s\text{)}[/latex]
- [latex]\text{MgO(}s\text{)}+2\text{HCl(}aq\text{)}\rightarrow{\text{MgCl}}_{2}\text{(}aq\text{)}+{\text{H}}_{2}\text{O(}l\text{)}[/latex]
- [latex]{\text{K}}_{3}\text{P(}s\text{)}+2{\text{O}}_{2}\text{(}g\text{)}\rightarrow{\text{K}}_{3}{\text{PO}}_{4}\text{(}s\text{)}[/latex]
- [latex]3\text{KOH(}aq\text{)}+{\text{H}}_{3}{\text{PO}}_{4}\text{(}aq\text{)}\rightarrow{\text{K}}_{3}{\text{PO}}_{4}\text{(}aq\text{)}+3{\text{H}}_{2}\text{O(}l\text{)}[/latex]
- Identify the atoms that are oxidized and reduced, the change in oxidation state for each, and the oxidizing and reducing agents in each of the following equations:
- [latex]\text{Mg(}s\text{)}+{\text{NiCl}}_{2}\text{(}aq\text{)}\rightarrow{\text{MgCl}}_{2}\text{(}aq\text{)}+\text{Ni(}s\text{)}[/latex]
- [latex]{\text{PCl}}_{3}\text{(}l\text{)}+{\text{Cl}}_{2}\text{(}g\text{)}\rightarrow{\text{PCl}}_{5}\text{(}s\text{)}[/latex]
- [latex]{\text{C}}_{2}{\text{H}}_{4}\text{(}g\text{)}+3{\text{O}}_{2}\text{(}g\text{)}\rightarrow 2{\text{CO}}_{2}\text{(}g\text{)}+2{\text{H}}_{2}\text{O(}g\text{)}[/latex]
- [latex]\text{Zn(}s\text{)}+{\text{H}}_{2}{\text{SO}}_{4}\text{(}aq\text{)}\rightarrow{\text{ZnSO}}_{4}\text{(}aq\text{)}+{\text{H}}_{2}\text{(}g\text{)}[/latex]
- [latex]2{\text{K}}_{2}{\text{S}}_{2}{\text{O}}_{3}\text{(}s\text{)}+{\text{I}}_{2}\text{(}s\text{)}\rightarrow{\text{K}}_{2}{\text{S}}_{4}{\text{O}}_{6}\text{(}s\text{)}+2\text{KI(}s\text{)}[/latex]
- [latex]3\text{Cu(}s\text{)}+8{\text{HNO}}_{3}\text{(}aq\text{)}\rightarrow 3\text{Cu}{\text{(}{\text{NO}}_{3}\text{)}}_{2}\text{(}aq\text{)}+2\text{NO(}g\text{)}+4{\text{H}}_{2}\text{O(}l\text{)}[/latex]
- Complete and balance the following acid-base equations:
- [latex]\ce{HCl}[/latex] gas reacts with solid [latex]\ce{Ca(OH)2}(s)[/latex].
- A solution of [latex]\ce{Sr(OH)2}[/latex] is added to a solution of [latex]\ce{HNO3}[/latex].
- Complete and balance the following acid-base equations:
- A solution of [latex]\ce{HClO4}[/latex] is added to a solution of [latex]\ce{LiOH}[/latex].
- Aqueous [latex]\ce{H2SO4}[/latex] reacts with [latex]\ce{NaOH}[/latex].
- [latex]\ce{Ba(OH)2}[/latex] reacts with [latex]\ce{HF}[/latex] gas.
- Complete and balance the following oxidation-reduction reactions, which give the highest possible oxidation state for the oxidized atoms.
- [latex]\text{Al(}s\text{)}+{\text{F}}_{2}\text{(}g\text{)}\rightarrow[/latex]
- [latex]\text{Al(}s\text{)}+{\text{CuBr}}_{2}\text{(}aq\text{)}\rightarrow[/latex] (single displacement)
- [latex]{\text{P}}_{4}\text{(}s\text{)}+{\text{O}}_{2}\text{(}g\text{)}\rightarrow[/latex]
- [latex]\text{Ca(}s\text{)}+{\text{H}}_{2}\text{O(}l\text{)}\rightarrow[/latex] (products are a strong base and a diatomic gas)
- Complete and balance the following oxidation-reduction reactions, which give the highest possible oxidation state for the oxidized atoms.
- [latex]\text{K(}s\text{)}+{\text{H}}_{2}\text{O(}l\text{)}\rightarrow[/latex]
- [latex]\text{Ba(}s\text{)}+\text{HBr(}aq\text{)}\rightarrow[/latex]
- [latex]\text{Sn(}s\text{)}+{\text{I}}_{2}\text{(}s\text{)}\rightarrow[/latex]
- Complete and balance the equations for the following acid-base neutralization reactions. If water is used as a solvent, write the reactants and products as aqueous ions. In some cases, there may be more than one correct answer, depending on the amounts of reactants used.
- [latex]\text{Mg}{\text{(OH)}}_{2}\text{(}s\text{)}+{\text{HClO}}_{4}\text{(}aq\text{)}\rightarrow[/latex]
- [latex]{\text{SO}}_{3}\text{(}g\text{)}+{\text{H}}_{2}\text{O(}l\text{)}\rightarrow\phantom{\rule{0.3em}{0ex}}\text{(assume an excess of water and that the product dissolves)}[/latex]
- [latex]\text{SrO(}s\text{)}+{\text{H}}_{2}{\text{SO}}_{4}\text{(}l\text{)}\rightarrow[/latex]
- When heated to 700–800 °C, diamonds, which are pure carbon, are oxidized by atmospheric oxygen. (They burn!) Write the balanced equation for this reaction.
- The military has experimented with lasers that produce very intense light when fluorine combines explosively with hydrogen. What is the balanced equation for this reaction?
- In a common experiment in the general chemistry laboratory, magnesium metal is heated in air to produce [latex]\ce{MgO}[/latex]. [latex]\ce{MgO}[/latex] is a white solid, but in these experiments it often looks gray, due to small amounts of [latex]\ce{Mg3N2}[/latex], a compound formed as some of the magnesium reacts with nitrogen. Write a balanced equation for each reaction.
- Lithium hydroxide may be used to absorb carbon dioxide in enclosed environments, such as manned spacecraft and submarines. Write an equation for the reaction that involves 2 mol of [latex]\ce{LiOH}[/latex] per 1 mol of [latex]\ce{CO2}[/latex]. (Hint: Water is one of the products.)
- Calcium propionate is sometimes added to bread to retard spoilage. This compound can be prepared by the reaction of calcium carbonate, [latex]\ce{CaCO3}[/latex], with propionic acid, [latex]\ce{C2H6CO2H}[/latex], which has properties similar to those of acetic acid. Write the balanced equation for the formation of calcium propionate.
- Complete and balance the equations of the following reactions, each of which could be used to remove hydrogen sulfide from natural gas:
- [latex]\text{Ca}{\text{(}\text{OH}\text{)}}_{2}\text{(}s\text{)}+{\text{H}}_{2}\text{S(}g\text{)}\rightarrow[/latex]
- [latex]{\text{Na}}_{2}{\text{CO}}_{3}\text{(}aq\text{)}+{\text{H}}_{2}\text{S(}g\text{)}\rightarrow[/latex]
- Copper(II) sulfide is oxidized by molecular oxygen to produce gaseous sulfur trioxide and solid copper(II) oxide. The gaseous product then reacts with liquid water to produce liquid hydrogen sulfate as the only product. Write the two equations which represent these reactions.
- Write balanced chemical equations for the reactions used to prepare each of the following compounds from the given starting material(s). In some cases, additional reactants may be required.
- solid ammonium nitrate from gaseous molecular nitrogen via a two-step process (first reduce the nitrogen to ammonia, then neutralize the ammonia with an appropriate acid)
- gaseous hydrogen bromide from liquid molecular bromine via a one-step redox reaction
- gaseous [latex]\ce{H2S}[/latex] from solid [latex]\ce{Zn}[/latex] and [latex]\ce{S}[/latex] via a two-step process (first a redox reaction between the starting materials, then reaction of the product with a strong acid)
- Calcium cyclamate [latex]\ce{Ca(C6H11NHSO3)2}[/latex] is an artificial sweetener used in many countries around the world but is banned in the United States. It can be purified industrially by converting it to the barium salt through reaction of the acid [latex]\ce{C6H11NHSO3H}[/latex] with barium carbonate, treatment with sulfuric acid (barium sulfate is very insoluble), and then neutralization with calcium hydroxide. Write the balanced equations for these reactions.
- Complete and balance each of the following half-reactions (steps 2–5 in half-reaction method):
- [latex]{\text{Sn}}^{\text{4+}}\text{(}aq\text{)}\rightarrow{\text{Sn}}^{\text{2+}}\text{(}aq\text{)}[/latex]
- [latex]{\left[\text{Ag}{\text{(}{\text{NH}}_{3}\text{)}}_{2}\right]}^{+}\text{(}aq\text{)}\rightarrow\text{Ag}\text{(}s\text{)}+{\text{NH}}_{3}\text{(}aq\text{)}[/latex]
- [latex]{\text{Hg}}_{2}{\text{Cl}}_{2}\text{(}s\text{)}\rightarrow\text{Hg}\text{(}l\text{)}+{\text{Cl}}^{-}\text{(}aq\text{)}[/latex]
- [latex]{\text{H}}_{2}\text{O(}l\text{)}\rightarrow{\text{O}}_{2}\text{(}\text{in acidic solution}\text{)}[/latex]
- [latex]{\text{IO}}_{3}{}^{-}\text{(}aq\text{)}\rightarrow{\text{I}}_{2}\text{(}s\text{)}[/latex]
- [latex]{\text{SO}}_{3}{}^{\text{2-}}\text{(}aq\text{)}\rightarrow{\text{SO}}_{4}{}^{\text{2-}}\text{(}aq\text{) (in acidic solution)}[/latex]
- [latex]{\text{MnO}}_{4}{}^{-}\text{(}aq\text{)}\rightarrow{\text{Mn}}^{\text{2+}}\text{(}aq\text{) (in acidic solution)}[/latex]
- [latex]{\text{Cl}}^{-}\text{(}aq\text{)}\rightarrow{\text{ClO}}_{3}{}^{-}\text{(}aq\text{) (in basic solution)}[/latex]
- Complete and balance each of the following half-reactions (steps 2–5 in half-reaction method):
- [latex]{\text{Cr}}^{\text{2+}}\text{(}aq\text{)}\rightarrow{\text{Cr}}^{\text{3+}}\text{(}aq\text{)}[/latex]
- [latex]\text{Hg(}l\text{)}+{\text{Br}}^{-}\text{(}aq\text{)}\rightarrow{\text{HgBr}}_{4}{}^{\text{2-}}\text{(}aq\text{)}[/latex]
- [latex]\text{ZnS(}s\text{)}\rightarrow\text{Zn(}s\text{)}+{\text{S}}^{\text{2-}}\text{(}aq\text{)}[/latex]
- [latex]{\text{H}}_{2}\text{(}g\text{)}\rightarrow{\text{H}}_{2}\text{O(}l\text{)}\text{(}\text{in basic solution}\text{)}[/latex]
- [latex]{\text{H}}_{2}\text{(}g\text{)}\rightarrow{\text{H}}_{3}{\text{O}}^{\text{+}}\text{(}aq\text{)}\text{(}\text{in acidic solution}\text{)}[/latex]
- [latex]{\text{NO}}_{3}{}^{-}\text{(}aq\text{)}\rightarrow{\text{HNO}}_{2}\text{(}aq\text{)}\text{(}\text{in acidic solution}\text{)}[/latex]
- [latex]{\text{MnO}}_{2}\text{(}s\text{)}\rightarrow{\text{MnO}}_{4}{}^{-}\text{(}aq\text{)}\text{(}\text{in basic solution}\text{)}[/latex]
- [latex]{\text{Cl}}^{-}\text{(}aq\text{)}\rightarrow{\text{ClO}}_{3}{}^{-}\text{(}aq\text{) (in acidic solution)}[/latex]
- Balance each of the following equations according to the half-reaction method:
- [latex]{\text{Sn}}^{\text{2+}}\text{(}aq\text{)}+{\text{Cu}}^{\text{2+}}\text{(}aq\text{)}\rightarrow{\text{Sn}}^{\text{4+}}\text{(}aq\text{)}+{\text{Cu}}^{+}\text{(}aq\text{)}[/latex]
- [latex]{\text{H}}_{2}\text{S(}g\text{)}+{\text{Hg}}_{2}{}^{\text{2+}}\text{(}aq\text{)}\rightarrow\text{H}g\text{(}l\text{)}+\text{S(}s\text{) (in acid)}[/latex]
- [latex]{\text{CN}}^{-}\text{(}aq\text{)}+{\text{ClO}}_{2}\text{(}aq\text{)}\rightarrow{\text{CNO}}^{-}\text{(}aq\text{)}+{\text{Cl}}^{-}\text{(}aq\text{) (in acid)}[/latex]
- [latex]{\text{Fe}}^{\text{2+}}\text{(}aq\text{)}+{\text{Ce}}^{\text{4+}}\text{(}aq\text{)}\rightarrow{\text{Fe}}^{\text{3+}}\text{(}aq\text{)}+{\text{Ce}}^{\text{3+}}\text{(}aq\text{)}[/latex]
- [latex]\text{HBrO(}aq\text{)}\rightarrow{\text{Br}}^{-}\text{(}aq\text{)}+{\text{O}}_{2}\text{(}g\text{) (in acid)}[/latex]
- Balance each of the following equations according to the half-reaction method:
- [latex]\text{Zn(}s\text{)}+{\text{NO}}_{3}{}^{-}\text{(}aq\text{)}\rightarrow{\text{Zn}}^{\text{2+}}\text{(}aq\text{)}+{\text{N}}_{2}\text{(}g\text{) (in acid)}[/latex]
- [latex]\text{Zn(}s\text{)}+{\text{NO}}_{3}{}^{-}\text{(}aq\text{)}\rightarrow{\text{Zn}}^{\text{2+}}\text{(}aq\text{)}+{\text{NH}}_{3}\text{(}aq\text{) (in base)}[/latex]
- [latex]\text{CuS(}s\text{)}+{\text{NO}}_{3}{}^{-}\text{(}aq\text{)}\rightarrow{\text{Cu}}^{\text{2+}}\text{(}aq\text{)}+\text{S(}s\text{)}+\text{NO(}g\text{) (in acid)}[/latex]
- [latex]{\text{NH}}_{3}\text{(}aq\text{)}+{\text{O}}_{2}\text{(}g\text{)}\rightarrow{\text{NO}}_{2}\text{(}g\text{) (gas phase)}[/latex]
- [latex]{\text{Cl}}_{2}\text{(}g\text{)}+{\text{OH}}^{-}\text{(}aq\text{)}\rightarrow{\text{Cl}}^{-}\text{(}aq\text{)}+{\text{ClO}}_{3}{}^{-}\text{(}aq\text{) (in base)}[/latex]
- [latex]{\text{H}}_{2}{\text{O}}_{2}\text{(}aq\text{)}+{\text{MnO}}_{4}{}^{-}\text{(}aq\text{)}\rightarrow{\text{Mn}}^{\text{2+}}\text{(}aq\text{)}+{\text{O}}_{2}\text{(}g\text{) (in acid)}[/latex]
- [latex]{\text{NO}}_{2}\text{(}g\text{)}\rightarrow{\text{NO}}_{3}{}^{-}\text{(}aq\text{)}+{\text{NO}}_{2}{}^{-}\text{(}aq\text{) (in base)}[/latex]
- [latex]{\text{Fe}}^{\text{3+}}\text{(}aq\text{)}+{\text{I}}^{-}\text{(}aq\text{)}\rightarrow{\text{Fe}}^{\text{2+}}\text{(}aq\text{)}+{\text{I}}_{2}\text{(}aq\text{)}[/latex]
- Balance each of the following equations according to the half-reaction method:
- [latex]{\text{MnO}}_{4}{}^{-}\text{(}aq\text{)}+{\text{NO}}_{2}{}^{-}\text{(}aq\text{)}\rightarrow{\text{MnO}}_{2}\text{(}s\text{)}+{\text{NO}}_{3}{}^{-}\text{(}aq\text{) (in base)}[/latex]
- [latex]{\text{MnO}}_{4}{}^{\text{2-}}\text{(}aq\text{)}\rightarrow{\text{MnO}}_{4}{}^{-}\text{(}aq\text{)}+{\text{MnO}}_{2}\text{(}s\text{) (in base)}[/latex]
- [latex]{\text{Br}}_{2}\text{(}l\text{)}+{\text{SO}}_{2}\text{(}g\text{)}\rightarrow{\text{Br}}^{-}\text{(}aq\text{)}+{\text{SO}}_{4}{}^{\text{2-}}\text{(}aq\text{) (in acid)}[/latex]
- Use the following equations to answer the next four questions:
- [latex]{\text{H}}_{2}\text{O(}s\text{)}\rightarrow{\text{H}}_{2}\text{O(}l\text{)}[/latex]
- [latex]{\text{Na}}^{+}\text{(}aq\text{)}+{\text{Cl}}^{-}\text{(}aq\text{)}{\text{Ag}}^{+}\text{(}aq\text{)}+{\text{NO}}_{3}{}^{-}\text{(}aq\text{)}\rightarrow\text{AgCl(}s\text{)}+{\text{Na}}^{+}\text{(}aq\text{)}+{\text{NO}}_{3}{}^{-}\text{(}aq\text{)}[/latex]
- [latex]{\text{CH}}_{3}\text{OH(}g\text{)}+{\text{O}}_{2}\text{(}g\text{)}\rightarrow{\text{CO}}_{2}\text{(}g\text{)}+{\text{H}}_{2}\text{O(}g\text{)}[/latex]
- [latex]2{\text{H}}_{2}\text{O(}l\text{)}\rightarrow 2{\text{H}}_{2}\text{(}g\text{)}+{\text{O}}_{2}\text{(}g\text{)}[/latex]
- [latex]{\text{H}}^{\text{+}}\text{(}aq\text{)}+{\text{OH}}^{-}\text{(}aq\text{)}\rightarrow{\text{H}}_{2}\text{O(}l\text{)}[/latex]
- Which equation describes a physical change?
- Which equation identifies the reactants and products of a combustion reaction?
- Which equation is not balanced?
- Which is a net ionic equation?
Show Selected Solutions
- The answers are as follows:
- oxidation-reduction (addition)
- acid-base (neutralization)
- oxidation-reduction (combustion)
- An oxidation-reduction reaction, because the oxidation state of the silver changes during the reaction
- The answers are as follows:
- [latex]\ce{H}[/latex] +1, [latex]\ce{P}[/latex] +5, [latex]\ce{O}[/latex] –2
- [latex]\ce{Al}[/latex] +3, [latex]\ce{H}[/latex] +1, [latex]\ce{O}[/latex] –2
- [latex]\ce{Se}[/latex] +4, [latex]\ce{O}[/latex] –2
- [latex]\ce{K}[/latex] +1, [latex]\ce{N}[/latex] +3, [latex]\ce{O}[/latex] –2
- [latex]\ce{In}[/latex] +3, [latex]\ce{S}[/latex] –2
- [latex]\ce{P}[/latex] +3, [latex]\ce{O}[/latex] –2
- The answers are as follows:
- acid-base
- oxidation-reduction
- oxidation-reduction
- acid-base
- oxidation-reduction
- acid-base
- The answers are as follows:
- [latex]\ce{2HCl} (g) + \ce{Ca(OH)2}(s) \longrightarrow \ce{CaCl2}(s) + 2\ce{H2O}(l)[/latex]
- [latex]\ce{Sr(OH)2}(aq) + \ce{2HNO3}(aq) \longrightarrow \ce{Sr(NO3)2}(aq) + 2\ce{H2O}(l)[/latex]
- The answers are as follows:
- [latex]\ce{2Al}(s) + 3\ce{F2}(g) \longrightarrow \ce{2AlF3}(s)[/latex]
- [latex]\ce{2Al}(s) + 3\ce{CuBr2}(aq) \longrightarrow \ce{3Cu}(s) + 2\ce{AlBr3}(aq)[/latex]
- [latex]\ce{P4}(s) + \ce{5O2}(g) \longrightarrow \ce{P4O_{10}}(s)[/latex]
- [latex]\ce{Ca}(s) + 2\ce{H2O}(l) \longrightarrow \ce{Ca(OH)2}(aq) + \ce{H2}(g)[/latex]
- The answers are as follows:
- [latex]\ce{Mg(OH)2}(s) + \ce{2HCIO4}(aq) \longrightarrow \ce{Mg^{2+}}(aq) + 2\ce{CIO4^-}(aq) + \ce{2H2O}(l)[/latex]
- [latex]\ce{SO3}(g) + \ce{2H2O}(l) \longrightarrow \ce{H3O^+} + \ce{HSO4^-}(aq)[/latex] (a solution of [latex]\ce{H2SO4}[/latex]
- [latex]\ce{SrO}(s) + \ce{H2SO4}(l) \longrightarrow \ce{SrSO4} + \ce{H2O}[/latex]
- [latex]\ce{H2}(g) + \ce{F2}(g) \longrightarrow \ce{2HF}(g)[/latex]
- [latex]\ce{2LiOH}(aq) + \ce{CO2} \longrightarrow \ce{Li2CO3}(aq) + \ce{H2O}(l)[/latex]
- The answers are as follows:
- [latex]\ce{Ca(OH)2}(g) + \ce{H2S}(g) \longrightarrow \ce{CaS}(s) + \ce{2H2O}(l)[/latex]
- [latex]\ce{Na2Co3}(aq) + \ce{H2S}(s) \longrightarrow \ce{Na2S}(aq) + \ce{CO2}(g) + \ce{H2O}(l)[/latex]
- The answers are as follows:
- [latex]\ce{N2}(g) + \ce{3H2}(g) \longrightarrow \ce{2NH3}(g)[/latex] and [latex]\ce{NH3}(g) + \ce{NHO3}(aq) \longrightarrow \ce{NH4NO3}(aq) \longrightarrow \ce{NH4NO3}(s)[/latex] (after drying)
- [latex]\ce{H2}(g)+\ce{Br2}(l) \longrightarrow \ce{2HBr}(g)[/latex]
- [latex]\ce{Zn}(s) + \ce{S}(s) \longrightarrow \ce{ZnS}(s)[/latex] and [latex]\ce{ZnS}(s) + \ce{2HCl}(aq) \longrightarrow \ce{ZnCl2}(aq) + \ce{H2S}(g)[/latex]
- The answers are as follows:
- [latex]\begin{array}{l} \ce{Sn^{4+}}(aq) \longrightarrow \ce{Sn^{2+}}(aq) \\ \ce{Sn^{4+}}(aq) + 2e^- \longrightarrow \ce{Sn^{2+}}(aq) \\ \end{array}[/latex]
- [latex]\begin{array}{l} \ce{[Ag(NH3)2]}(aq) \longrightarrow \ce{Ag}(s) + \ce{2NH3}(aq) \\ \ce{[Ag(NH3)2]}(aq) + e^- \longrightarrow \ce{Ag}(s) + \ce{2NH3}(aq) \\ \end{array}[/latex]
- [latex]\begin{array}{l} \ce{Hg2Cl2}(s) \longrightarrow \ce{Hg}(l) + \ce{Cl^-}(aq) \\ \ce{Hg2Cl2}(s) \longrightarrow \ce{2Hg}(l) + \ce{2Cl^-}(aq) \\ \ce{Hg2Cl2}(s) + 2e^- \longrightarrow \ce{2Hg}(l) + \ce{2Cl^-}(aq) \\ \end{array}[/latex]
- [latex]\begin{array}{l} \ce{2H2O}(l) \longrightarrow \ce{O2}(g) \\ \ce{2H2O}(l) \longrightarrow \ce{O2}(g) + \ce{4H^+}(aq) \\ \ce{2H2O}(l) \longrightarrow \ce{O2}(g) + \ce{4H^+}(aq) + 4e^- \\ \end{array}[/latex]
- [latex]\begin{array}{l} \ce{IO3^-}(aq) \longrightarrow \ce{I2}(s) \\ \ce{2IO3^-}(aq) \longrightarrow \ce{I2}(s) \\ \ce{2IO3^-}(aq) \longrightarrow \ce{I2}(s) + \ce{6H2O}(l) \\ \ce{12H^+}(aq) + \ce{2IO3^-}(aq) \longrightarrow \ce{I2}(s) + \ce{6H2O}(l) \\ \ce{12H^+}(aq) + \ce{12OH^-}(aq) \ce{2IO3^-}(aq) \longrightarrow \ce{I2}(s) + \ce{6H2O}(l) + \ce{12OH^-}(aq) \\ \ce{12H2O}(aq) + \ce{2IO3^-}(aq) \longrightarrow \ce{I2}(s) + \ce{6H2O}(l) + \ce{12OH^-}(aq) \\ \ce{6H2O}(aq) + \ce{2IO3^-}(aq) \longrightarrow \ce{I2}(s) + \ce{12OH^-}(aq) \\ \ce{6H2O}(aq) + \ce{2IO3^-}(aq) + 10e^- \longrightarrow \ce{I2}(s) + \ce{12OH^-}(aq) \\ \end{array}[/latex]
- [latex]\begin{array}{l} \ce{SO3^{2-}}(aq) \longrightarrow \ce{SO4^{2-}}(aq) \\ \ce{H2O}(l) + \ce{SO3^{2-}} \longrightarrow \ce{SO4^{2-}}(aq) + \ce{2H^+}(aq) \\ \ce{H2O}(l) + \ce{SO3^{2-}} \longrightarrow \ce{SO4^{2-}}(aq) + \ce{2H^+}(aq) + 2e^- \\ \end{array}[/latex]
- [latex]\begin{array}{l} \ce{MnO4^-}(aq) \longrightarrow \ce{Mn^{2+}}(aq) \\ \ce{MnO4^-}(aq) \longrightarrow \ce{Mn^{2+}}(aq) + \ce{4H2O}(l). \\ \ce{8H^+}(aq) + \ce{MnO4^-}(aq) \longrightarrow \ce{Mn^{2+}}(aq) + \ce{4H2O}(l). \\ \ce{8H^+}(aq) + \ce{MnO4^-}(aq) + 5e^- \longrightarrow \ce{Mn^{2+}}(aq) + \ce{4H2O}(l). \\ \end{array}[/latex]
- [latex]\begin{array}{l} \ce{Cl^-}(aq) \longrightarrow \ce{ClO3^-}(aq) \\ \ce{3H2O}(l) + \ce{Cl^-}(aq) \longrightarrow \ce{ClO3^-}(aq) \\ \ce{3H2O}(l) + \ce{Cl^-}(aq) \longrightarrow \ce{ClO3^-}(aq) + \ce{6H^+}(aq) \\ \ce{3H2O}(l) + \ce{Cl^-}(aq) + \ce{6OH^-}(aq) \longrightarrow \ce{ClO3^-}(aq) + \ce{6H^+}(aq) + \ce{6OH^-}(aq) \\ \ce{3H2O}(l) + \ce{Cl^-}(aq) + \ce{6OH^-}(aq) \longrightarrow \ce{ClO3^-}(aq) + \ce{6H2O}(l) \\ \ce{Cl^-}(aq) + \ce{6OH^-}(aq) \longrightarrow \ce{ClO3^-}(aq) + \ce{3H2O}(l) + 6e^- \\ \end{array}[/latex]
- The answers are as follows:
- [latex]\ce{Sn^{2-}}(aq) + \ce{2Cu^{2+}}(aq) \longrightarrow \ce{Sn^{4+}}(aq) + \ce{2Cu^+}[/latex]
- [latex]\ce{H2S}(g) + \ce{Hg2^{2+}}(aq) + \ce{2H2O}(l) \longrightarrow 2\ce{Hg}(l) + \ce{S}(s) + \ce{2H3O^+}(aq)[/latex]
- [latex]\ce{5CN^-}(aq) + 2\ce{ClO2}(aq) + \ce{3H2O}(l) \longrightarrow 5\ce{CNO^-}(aq) + \ce{2Cl^-}(aq) + 2\ce{2H3O^+}(aq)[/latex]
- [latex]\ce{Fe^{2+}}(aq) + \ce{Ce^{4+}}(aq) \longrightarrow \ce{Fe^{3+}}(aq) + \ce{Ce^3+}(aq)[/latex]
- [latex]\ce{2HBrO}(aq) + \ce{2H2O}(l) \longrightarrow \ce{2H3O^+}(aq) + \ce{2Br^-}(aq) + \ce{O2}(g)[/latex]
- The answers are as follows:
- [latex]\ce{2MnO4^-}(aq) + \ce{3NO2^-}(aq) + \ce{H2O}(l) \longrightarrow \ce{2mnO2^-}(s) + \ce{3NO3^-}(aq) + \ce{2OH^-}(aq)[/latex]
- [latex]\ce{3MnO4^{2-}}(aq) + \ce{2H2O}(l) \longrightarrow \ce{2MnO4^-}(aq) + \ce{4OH^-}(aq) + \ce{MnO2}(s) \text{ (in base)}[/latex]
- [latex]\ce{Br2}(l) + \ce{SO2}(g) + \ce{2H2O}(l) \longrightarrow \ce{4H^+}(aq) + \ce{2Br^-}(aq) + \ce{SO4^{2-}}(aq)[/latex]
8.4 Reaction Yields [Go to section 8.4]
- Freon-12, [latex]\ce{CCl2F2}[/latex], is prepared from [latex]\ce{CCl4}[/latex] by reaction with [latex]\ce{HF}[/latex]. The other product of this reaction is [latex]\ce{HCl}[/latex]. Outline the steps needed to determine the percent yield of a reaction that produces 12.5 g of [latex]\ce{CCl2F2}[/latex] from 32.9 g of [latex]\ce{CCl4}[/latex]. Freon-12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone and has a very long lifetime in the atmosphere. Determine the percent yield.
- The following quantities are placed in a container: 1.5 × 1024 atoms of hydrogen, 1.0 mol of sulfur, and 88.0 g of diatomic oxygen.
- What is the total mass in grams for the collection of all three elements?
- What is the total number of moles of atoms for the three elements?
- If the mixture of the three elements formed a compound with molecules that contain two hydrogen atoms, one sulfur atom, and four oxygen atoms, which substance is consumed first?
- How many atoms of each remaining element would remain unreacted in the change described in (c)?
- Outline the steps needed to solve the following problem, then do the calculations. Ether, [latex]\ce{(C2H5)2O}[/latex], which was originally used as an anesthetic but has been replaced by safer and more effective medications, is prepared by the reaction of ethanol with sulfuric acid. [latex]2{\text{C}}_{2}{\text{H}}_{5}\text{OH}+{\text{H}}_{2}{\text{SO}}_{4}\rightarrow{\text{(}{\text{C}}_{2}{\text{H}}_{5}\text{)}}_{2}\text{O}+{\text{H}}_{2}{\text{SO}}_{4}\cdot {\text{H}}_{2}\text{O}[/latex] What is the percent yield of ether if 1.17 L (d = 0.7134 g/mL) is isolated from the reaction of 1.500 L of [latex]\ce{C2H5OH}[/latex] (d = 0.7894 g/mL)?
- Which of the postulates of Dalton's atomic theory explains why we can calculate a theoretical yield for a chemical reaction?
- Outline the steps needed to determine the limiting reactant when 0.50 g of [latex]\ce{Cr}[/latex] and 0.75 g of [latex]\ce{H3PO4}[/latex] react according to the following chemical equation? [latex]2\text{Cr}+2{\text{H}}_{3}{\text{PO}}_{4}\rightarrow 2{\text{CrPO}}_{4}+3{\text{H}}_{2}[/latex] Determine the limiting reactant.
- A sample of 0.53 g of carbon dioxide was obtained by heating 1.31 g of calcium carbonate. What is the percent yield for this reaction? [latex]{\text{CaCO}}_{3}\text{(}s\text{)}\rightarrow\text{CaO}\text{(}s\text{)}+{\text{CO}}_{2}\text{(}s\text{)}[/latex]
- Uranium can be isolated from its ores by dissolving it as [latex]\ce{UO2(NO3)2}[/latex], then separating it as solid [latex]\ce{UO2(C2O4)}[/latex] [latex]\cdot[/latex] [latex]\ce{3H2O}[/latex]. Addition of 0.4031 g of sodium oxalate, [latex]\ce{Na2C2O4}[/latex], to a solution containing 1.481 g of uranyl nitrate, [latex]\ce{UO2(NO2)2}[/latex], yields 1.073 g of solid [latex]\ce{UO2(C2O4)}[/latex] [latex]\cdot[/latex] [latex]\ce{3H2O}[/latex]. [latex]{\text{Na}}_{2}{\text{C}}_{2}{\text{O}}_{4}+{\text{UO}}_{2}{\text{(}{\text{NO}}_{3}\text{)}}_{2}+3{\text{H}}_{2}\text{O}\rightarrow{\text{UO}}_{2}\text{(}{\text{C}}_{2}{\text{O}}_{4}\text{)}\cdot 3{\text{H}}_{2}\text{O}+2{\text{NaNO}}_{3}[/latex] Determine the limiting reactant and the percent yield of this reaction.
- Citric acid, [latex]\ce{C6H8O7}[/latex], a component of jams, jellies, and fruity soft drinks, is prepared industrially via fermentation of sucrose by the mold Aspergillus niger. The equation representing this reaction is [latex]{\text{C}}_{12}{\text{H}}_{22}{\text{O}}_{11}+{\text{H}}_{2}\text{O}+3{\text{O}}_{2}\rightarrow 2{\text{C}}_{6}{\text{H}}_{8}{\text{O}}_{7}+4{\text{H}}_{2}\text{O}[/latex]. What mass of citric acid is produced from exactly 1 metric ton (1.000 × 103 kg) of sucrose if the yield is 92.30%?
- How many molecules of the sweetener saccharin can be prepared from 30 [latex]\ce{C}[/latex] atoms, 25 [latex]\ce{H}[/latex] atoms, 12 [latex]\ce{O}[/latex] atoms, 8 [latex]\ce{S}[/latex] atoms, and 14 [latex]\ce{N}[/latex] atoms?
- In a laboratory experiment, the reaction of 3.0 mol of [latex]\ce{H2}[/latex] with 2.0 mol of [latex]\ce{I2}[/latex] produced 1.0 mol of [latex]\ce{HI}[/latex]. Determine the theoretical yield in grams and the percent yield for this reaction.
- What is the limiting reactant when 15.0 g of propane, [latex]\ce{C3H8}[/latex], and 60.0 g of oxygen react ([latex]\ce{O2}[/latex])?
- The phosphorus pentoxide used to produce phosphoric acid for cola soft drinks is prepared by burning phosphorus in oxygen.
- What is the limiting reactant when 0.200 mol of [latex]\ce{P4}[/latex] and 0.200 mol of [latex]\ce{O2}[/latex] react according to [latex]{\text{P}}_{4}+5{\text{O}}_{2}\rightarrow{\text{P}}_{4}{\text{O}}_{10}[/latex]
- Calculate the percent yield if 10.0 g of [latex]\ce{P4O_{10}}[/latex] is isolated from the reaction.
8.5 Solutions and Molarity [Go to section 8.5]
- Explain what changes and what stays the same when 1.00 L of a solution of [latex]\ce{NaCl}[/latex] is diluted to 1.80 L.
- Consider this question: What is the mass of the solute in 0.500 L of 0.30 M glucose, [latex]\ce{C6H_{12}O6}[/latex], used for intravenous injection?
- Outline the steps necessary to answer the question.
- Answer the question.
- What does it mean when we say that a 200-mL sample and a 400-mL sample of a solution of salt have the same molarity? In what ways are the two samples identical? In what ways are these two samples different
- Consider this question: What is the molarity of [latex]\ce{KMnO4}[/latex] in a solution of 0.0908 g of [latex]\ce{KMnO4}[/latex] in 0.500 L of solution?
- Outline the steps necessary to answer the question.
- Answer the question.
- Determine the molarity of each of the following solutions:
- 1.457 mol [latex]\ce{KCl}[/latex] in 1.500 L of solution
- 0.515 g of [latex]\ce{H2SO4}[/latex] in 1.00 L of solution
- 20.54 g of [latex]\ce{Al(NO3)3}[/latex] in 1575 mL of solution
- 2.76 kg of [latex]\ce{CuSO4} \cdot \ce{5H2O}[/latex] in 1.45 L of solution
- 0.005653 mol of [latex]\ce{Br2}[/latex] in 10.00 mL of solution
- 0.000889 g of glycine, [latex]\ce{C2H5NO2}[/latex], in 1.05 mL of solution
- Calculate the molarity of each of the following solutions:
- 0.195 g of cholesterol, [latex]\ce{C_{27}H_{46}O}[/latex], in 0.100 L of serum, the average concentration of cholesterol in human serum
- 4.25 g of [latex]\ce{NH3}[/latex] in 0.500 L of solution, the concentration of [latex]\ce{NH3}[/latex] in household ammonia
- 1.49 kg of isopropyl alcohol, [latex]\ce{C3H7OH}[/latex], in 2.50 L of solution, the concentration of isopropyl alcohol in rubbing alcohol
- 0.029 g of [latex]\ce{I2}[/latex] in 0.100 L of solution, the solubility of [latex]\ce{I2}[/latex] in water at 20 °C
- Consider this question: What is the mass of solute in 200.0 L of a 1.556-M solution of [latex]\ce{KBr}[/latex]?
- Outline the steps necessary to answer the question.
- Answer the question.
- There is about 1.0 g of calcium, as [latex]\ce{Ca^2+}[/latex], in 1.0 L of milk. What is the molarity of [latex]\ce{Ca^2+}[/latex] in milk?
- Calculate the number of moles and the mass of the solute in each of the following solutions:
- 325 mL of [latex]8.23\times {10}^{-5}M\text{ KI}[/latex], a source of iodine in the diet
- 75.0 mL of [latex]2.2\times {10}^{-5}M{\text{ H}}_{2}{\text{SO}}_{4}[/latex], a sample of acid rain
- 0.2500 L of [latex]0.1135M\text{ K}_{2}\text{CrO}_{4}[/latex], an analytical reagent used in iron assays
- 10.5 L of [latex]3.716M\text{ }(\text{NH}_{4})_{2}\text{SO}_{4}[/latex], a liquid fertilizer
- If 0.1718 L of a 0.3556-M [latex]\ce{C3H7OH}[/latex] solution is diluted to a concentration of 0.1222 M, what is the volume of the resulting solution?
- Consider this question: What is the molarity of [latex]\ce{HCl}[/latex] if 35.23 mL of a solution of [latex]\ce{HCl}[/latex] contain 0.3366 g of [latex]\ce{HCl}[/latex]?
- Outline the steps necessary to answer the question.
- Answer the question.
- What volume of a 0.33-M [latex]\ce{C_{12}H_{22}O_{11}}[/latex] solution can be diluted to prepare 25 mL of a solution with a concentration of 0.025 M?
- Calculate the molarity of each of the following solutions:
- 293 g [latex]\ce{HCl}[/latex] in 666 mL of solution, a concentrated [latex]\ce{HCl}[/latex] solution
- 2.026 g [latex]\ce{FeCl3}[/latex] in 0.1250 L of a solution used as an unknown in general chemistry laboratories
- 0.001 mg [latex]\ce{Cd^2+}[/latex] in 0.100 L, the maximum permissible concentration of cadmium in drinking water
- 0.0079 g [latex]\ce{C7H5SNO3}[/latex] in one ounce (29.6 mL), the concentration of saccharin in a diet soft drink.
- A 2.00-L bottle of a solution of concentrated [latex]\ce{HCl}[/latex] was purchased for the general chemistry laboratory. The solution contained 868.8 g of [latex]\ce{HCl}[/latex]. What is the molarity of the solution?
- What volume of a 1.00-M [latex]\ce{Fe(NO3)3}[/latex] solution can be diluted to prepare 1.00 L of a solution with a concentration of 0.250 M?
- If 4.12 L of a 0.850 M-[latex]\ce{H3PO4}[/latex] solution is be diluted to a volume of 10.00 L, what is the concentration the resulting solution?
- What is the concentration of the [latex]\ce{NaCl}[/latex] solution that results when 0.150 L of a 0.556-M solution is allowed to evaporate until the volume is reduced to 0.105 L?
- What is the final concentration of the solution produced when 225.5 mL of a 0.09988-M solution of [latex]\ce{Na2CO3}[/latex] is allowed to evaporate until the solution volume is reduced to 45.00 mL?
- An experiment in a general chemistry laboratory calls for a 2.00-M solution of [latex]\ce{HCl}[/latex]. How many mL of 11.9 M [latex]\ce{HCl}[/latex] would be required to make 250 mL of 2.00 M [latex]\ce{HCl}[/latex]?
- The US Environmental Protection Agency (EPA) places limits on the quantities of toxic substances that may be discharged into the sewer system. Limits have been established for a variety of substances, including hexavalent chromium, which is limited to 0.50 mg/L. If an industry is discharging hexavalent chromium as potassium dichromate ([latex]\ce{K2Cr2O7}[/latex]), what is the maximum permissible molarity of that substance?
Show Selected Solutions
- The mass and number of moles of NaCl stay the same. The volume of the solution and the concentration of [latex]\ce{NaCl}[/latex] change.
- When a 200-mL and a 400-mL sample have the same concentration, both solutions have the same amount of salt per unit volume. The solutions are different in that the 400 mL solution contains twice as much salt.
- The answers are as follows:
- 0.9713 M
- 0.00525 M
- 0.06123 M
- 7.62 M
- 0.5653 M
- 0.0113 M
- The answers are as follows:
- Determine the number of moles of [latex]\ce{KBr}[/latex] in 200.0 L of a 1.556-M solution. Determine the formula mass of [latex]\ce{KBr}[/latex]. Then determine the mass of [latex]\ce{KBr}[/latex] from the number of moles and its formula mass
- Mass ([latex]\ce{KBr}[/latex]) = 3.703 × 104 g
- The answers are as follows:
- [latex]2.67 × 10^{-5}\cancel{\text{ mol } \ce{KI}} × \frac{166.0028 \text{ g}}{1 \cancel{ mol KI}} = 4.43 × 10^{-3} \text{ g }\ce{KI}[/latex]
- [latex]1.6 × 10^{-6}\cancel{\text{ mol }\ce{H2SO4}} × \frac{98.079 \text{ g}}{1 \cancel{\text{ mol }\ce{H2SO4}}} = 1.6 × 10^{-6} \text{ g } \ce{H2SO4}[/latex]
- [latex]0.02838 \cancel{\text{ mol } \ce{K2CrO4}} × \frac{194.1903 \text{g}}{1 \cancel{\text{ mol } \ce{K2CrO4}}} = 5.511 \text{ g } \ce{K2CrO4}[/latex]
- [latex]\text{mol } \ce{(NH4)2SO4} = 10.5 \text{ L } × 3.716 M = 39.0 \cancel{\text{ mol } \ce{(NH4)2SO4}} × \frac{132.141 \text{ g }}{1 \cancel{\text{ mol } \ce{(NH4)2SO4}}} = 5.15 × 10^3 \text{ g } \ce{(NH4)2SO4}[/latex]
- The answers are as follows:
- Determine the molar mass of [latex]\ce{HCl}[/latex]. Determine the number of moles of [latex]\ce{HCl}[/latex] in the solution. From the number of moles and the volume of solution, determine the molarity.
- 0.2620 M
- The answers are as follows:
- 12.1 M
- 0.09992 M
- 9 × 10-8 M
- 1.5 × 10-3 M
- 0.250 L
- 0.794 M
- 42.0 mL
8.6 Other Units for Solution Concentrations [Go to section 8.6]
- What mass of [latex]\ce{HCl}[/latex] is contained in 45.0 mL of an aqueous [latex]\ce{HCl}[/latex] solution that has a density of 1.19 g cm[latex]^{–3}[/latex] and contains 37.21% [latex]\ce{HCl}[/latex] by mass?
- In Canada and the United Kingdom, devices that measure blood glucose levels provide a reading in millimoles per liter. If a measurement of 5.3 mM is observed, what is the concentration of glucose ([latex]\ce{C6H12O6}[/latex]) in mg/dL?
- The level of mercury in a stream was suspected to be above the minimum considered safe (1 part per billion by weight). An analysis indicated that the concentration was 0.68 parts per billion. Assume a density of 1.0 g/mL and calculate the molarity of mercury in the stream.
- A throat spray is 1.40% by mass phenol, [latex]\ce{C6H5OH}[/latex], in water. If the solution has a density of 0.9956 g/mL, calculate the molarity of the solution.
- A cough syrup contains 5.0% ethyl alcohol, [latex]\ce{C2H5OH}[/latex], by mass. If the density of the solution is 0.9928 g/mL, determine the molarity of the alcohol in the cough syrup.
- Find the molarity of a 40.0% by mass aqueous solution of sulfuric acid, [latex]\ce{H2SO4}[/latex], for which the density is 1.3057 g/mL.
8.7 Quantitative Chemical Analysis [Go to section 8.7]
- What volume of a 0.3300-M solution of sodium hydroxide would be required to titrate 15.00 mL of 0.1500 M oxalic acid? [latex]{\text{C}}_{2}{\text{O}}_{4}{\text{H}}_{2}\text{(}aq\text{)}+2\text{NaOH}\text{(}aq\text{)}\rightarrow{\text{Na}}_{2}{\text{C}}_{2}{\text{O}}_{4}\text{(}aq\text{)}+2{\text{H}}_{2}\text{O}\text{(}l\text{)}[/latex]
- What volume of 0.0105-M [latex]\ce{HBr}[/latex] solution is be required to titrate 125 mL of a 0.0100-M [latex]\ce{Ca(OH)2}[/latex] solution?[latex]\text{Ca}{\text{(}\text{OH}\text{)}}_{2}\text{(}aq\text{)}+2\text{HBr}\text{(}aq\text{)}\rightarrow{\text{CaBr}}_{2}\text{(}aq\text{)}+2{\text{H}}_{2}\text{O}\text{(}l\text{)}[/latex]
- A sample of solid calcium hydroxide, [latex]\ce{Ca(OH)2}[/latex], is allowed to stand in water until a saturated solution is formed. A titration of 75.00 mL of this solution with 5.00 × 10-2 M [latex]\ce{HCl}[/latex] requires 36.6 mL of the acid to reach the end point. [latex]\text{Ca}{\text{(}\text{OH}\text{)}}_{2}\text{(}aq\text{)}+2\text{HCl}\text{(}aq\text{)}\rightarrow{\text{CaCl}}_{2}\text{(}aq\text{)}+2{\text{H}}_{2}\text{O}\text{(}l\text{)}[/latex] The molarity? What is the solubility of [latex]\ce{Ca(OH)2}[/latex] in grams per liter of solution?
- What is the concentration of [latex]\ce{NaCl}[/latex] in a solution if titration of 15.00 mL of the solution with 0.2503 M [latex]\ce{AgNO3}[/latex] requires 20.22 mL of the [latex]\ce{AgNO3}[/latex] solution to reach the end point? [latex]{\text{AgNO}}_{3}\text{(}aq\text{)}+\text{NaCl}\text{(}aq\text{)}\rightarrow\text{AgCl}\text{(}s\text{)}+{\text{NaNO}}_{3}\text{(}aq\text{)}[/latex]
- How many milliliters of a 0.1500-M solution of [latex]\ce{KOH}[/latex] will be required to titrate 40.00 mL of a 0.0656-M solution of [latex]\ce{H3PO4}[/latex]? [latex]{\text{H}}_{3}{\text{PO}}_{4}\text{(}aq\text{)}+2\text{KOH}\text{(}aq\text{)}\rightarrow{\text{K}}_{2}{\text{HPO}}_{4}\text{(}aq\text{)}+2{\text{H}}_{2}\text{O}\text{(}l\text{)}[/latex]
- Potatoes can be peeled commercially by soaking them in a 3-M to 6-M solution of sodium hydroxide, then removing the loosened skins by spraying them with water. Does a sodium hydroxide solution have a suitable concentration if titration of 12.00 mL of the solution requires 30.6 mL of 1.65 M [latex]\ce{HCI}[/latex] to reach the end point?
- The reaction of [latex]\ce{WCl6}[/latex] with Al at ~400 °C gives black crystals of a compound containing only tungsten and chlorine. A sample of this compound, when reduced with hydrogen, gives 0.2232 g of tungsten metal and hydrogen chloride, which is absorbed in water. Titration of the hydrochloric acid thus produced requires 46.2 mL of 0.1051 M [latex]\ce{NaOH}[/latex] to reach the end point. What is the empirical formula of the black tungsten chloride?
- The principal component of mothballs is naphthalene, a compound with a molecular mass of about 130 amu, containing only carbon and hydrogen. A 3.000-mg sample of naphthalene burns to give 10.3 mg of [latex]\ce{CO2}[/latex]. Determine its empirical and molecular formulas.
- What mass of sodium bicarbonate ([latex]\ce{NaHCO3}[/latex]) is required to react completely with 500 mL of 0.855 M acetic acid ([latex]\ce{C2H4O2}[/latex])? The balanced reaction is:[latex]\ce{C2H4O2} (aq) + \ce{NaHCO3} (aq) \longrightarrow \ce{NaC2H3O2} (aq) + \ce{H2O} (l) + \ce{CO2} (g)[/latex]
- Sodium bicarbonate (baking soda), [latex]\ce{NaHCO3}[/latex], can be purified by dissolving it in hot water (60 °C), filtering to remove insoluble impurities, cooling to 0 °C to precipitate solid [latex]\ce{NaHCO3}[/latex], and then filtering to remove the solid, leaving soluble impurities in solution. Any [latex]\ce{NaHCO3}[/latex] that remains in solution is not recovered. The solubility of [latex]\ce{NaHCO3}[/latex] in hot water of 60 °C is 164 g L. Its solubility in cold water of 0 °C is 69 g/L. What is the percent yield of [latex]\ce{NaHCO3}[/latex] when it is purified by this method?
(also, reductant) substance that brings about the reduction of another substance, and in the process becomes oxidized
base that reacts completely when dissolved in water to yield hydroxide ions
reaction between an acid and a base to produce salt and water
acid that reacts completely when dissolved in water to yield hydronium ions
chemical equation in which only those dissolved ionic reactants and products that undergo a chemical or physical change are represented (excludes spectator ions)

