Chapter 1. Temperature and Heat
1.5 Phase Changes
Learning Objectives
By the end of this section, you will be able to:
- Describe phase transitions and equilibrium between phases
- Solve problems involving latent heat
- Solve calorimetry problems involving phase changes
Phase transitions play an important theoretical and practical role in the study of heat flow. In melting (or “fusion”), a solid turns into a liquid; the opposite process is freezing. In evaporation, a liquid turns into a gas; the opposite process is condensation.
A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or condenses at its boiling point. These temperatures depend on pressure. High pressure favors the denser form, so typically, high pressure raises the melting point and boiling point, and low pressure lowers them. For example, the boiling point of water is [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] at 1.00 atm. At higher pressure, the boiling point is higher, and at lower pressure, it is lower. The main exception is the melting and freezing of water, discussed in the next section.
Phase Diagrams
The phase of a given substance depends on the pressure and temperature. Thus, plots of pressure versus temperature showing the phase in each region provide considerable insight into thermal properties of substances. Such a pT graph is called a phase diagram.
Figure 1.12 shows the phase diagram for water. Using the graph, if you know the pressure and temperature, you can determine the phase of water. The solid curves—boundaries between phases—indicate phase transitions, that is, temperatures and pressures at which the phases coexist. For example, the boiling point of water is [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] at 1.00 atm. As the pressure increases, the boiling temperature rises gradually to [latex]374\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] at a pressure of 218 atm. A pressure cooker (or even a covered pot) cooks food faster than an open pot, because the water can exist as a liquid at temperatures greater than [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] without all boiling away. (As we’ll see in the next section, liquid water conducts heat better than steam or hot air.) The boiling point curve ends at a certain point called the critical point—that is, a critical temperature, above which the liquid and gas phases cannot be distinguished; the substance is called a supercritical fluid. At sufficiently high pressure above the critical point, the gas has the density of a liquid but does not condense. Carbon dioxide, for example, is supercritical at all temperatures above [latex]31.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex]. Critical pressure is the pressure of the critical point.
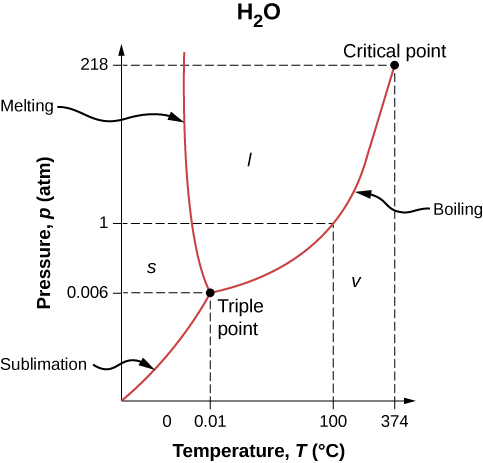
Similarly, the curve between the solid and liquid regions in Figure 1.12 gives the melting temperature at various pressures. For example, the melting point is [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}\phantom{\rule{0.2em}{0ex}}[/latex] at 1.00 atm, as expected. Water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. That is, the melting temperature of ice falls with increased pressure, as the phase diagram shows. For example, when a car is driven over snow, the increased pressure from the tires melts the snowflakes; afterwards, the water refreezes and forms an ice layer.
As you learned in the earlier section on thermometers and temperature scales, the triple point is the combination of temperature and pressure at which ice, liquid water, and water vapor can coexist stably—that is, all three phases exist in equilibrium. For water, the triple point occurs at [latex]273.16\phantom{\rule{0.2em}{0ex}}\text{K}\phantom{\rule{0.2em}{0ex}}\left(0.01\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}\right)[/latex] and 611.2 Pa; that is a more accurate calibration temperature than the melting point of water at 1.00 atm, or [latex]273.15\phantom{\rule{0.2em}{0ex}}\text{K}\phantom{\rule{0.2em}{0ex}}\left(0.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}\right)[/latex].
At pressures below that of the triple point, there is no liquid phase; the substance can exist as either gas or solid. For water, there is no liquid phase at pressures below 0.00600 atm. The phase change from solid to gas is called sublimation. You may have noticed that snow can disappear into thin air without a trace of liquid water, or that ice cubes can disappear in a freezer. Both are examples of sublimation. The reverse also happens: Frost can form on very cold windows without going through the liquid stage. Figure 1.13 shows the result, as well as showing a familiar example of sublimation. Carbon dioxide has no liquid phase at atmospheric pressure. Solid [latex]{\text{CO}}_{2}[/latex] is known as dry ice because instead of melting, it sublimes. Its sublimation temperature at atmospheric pressure is [latex]-78\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex]. Certain air fresheners use the sublimation of a solid to spread a perfume around a room. Some solids, such as osmium tetroxide, are so toxic that they must be kept in sealed containers to prevent human exposure to their sublimation-produced vapors.

Equilibrium
At the melting temperature, the solid and liquid phases are in equilibrium. If heat is added, some of the solid will melt, and if heat is removed, some of the liquid will freeze. The situation is somewhat more complex for liquid-gas equilibrium. Generally, liquid and gas are in equilibrium at any temperature. We call the gas phase a vapor when it exists at a temperature below the boiling temperature, as it does for water at [latex]20.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex]. Liquid in a closed container at a fixed temperature evaporates until the pressure of the gas reaches a certain value, called the vapor pressure, which depends on the gas and the temperature. At this equilibrium, if heat is added, some of the liquid will evaporate, and if heat is removed, some of the gas will condense; molecules either join the liquid or form suspended droplets. If there is not enough liquid for the gas to reach the vapor pressure in the container, all the liquid eventually evaporates.
If the vapor pressure of the liquid is greater than the total ambient pressure, including that of any air (or other gas), the liquid evaporates rapidly; in other words, it boils. Thus, the boiling point of a liquid at a given pressure is the temperature at which its vapor pressure equals the ambient pressure. Liquid and gas phases are in equilibrium at the boiling temperature (Figure 1.14). If a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the same rate without net change in their amounts.
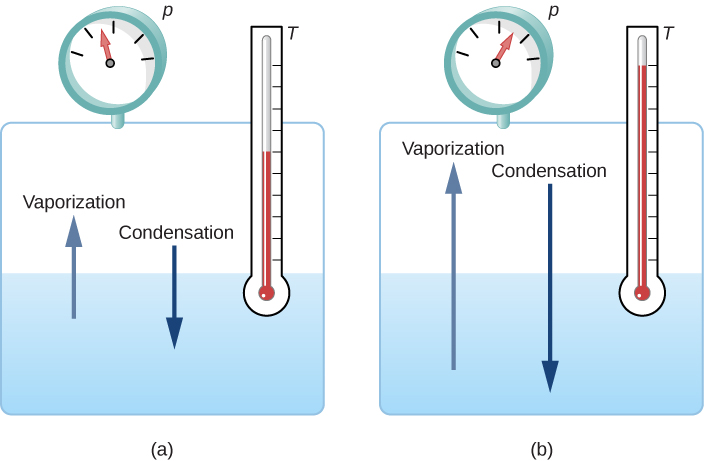
For water, [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] is the boiling point at 1.00 atm, so water and steam should exist in equilibrium under these conditions. Why does an open pot of water at [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] boil completely away? The gas surrounding an open pot is not pure water: it is mixed with air. If pure water and steam are in a closed container at [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] and 1.00 atm, they will coexist—but with air over the pot, there are fewer water molecules to condense, and water boils away. Another way to see this is that at the boiling point, the vapor pressure equals the ambient pressure. However, part of the ambient pressure is due to air, so the pressure of the steam is less than the vapor pressure at that temperature, and evaporation continues. Incidentally, the equilibrium vapor pressure of solids is not zero, a fact that accounts for sublimation.
Check Your Understanding
Explain why a cup of water (or soda) with ice cubes stays at [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C,}[/latex] even on a hot summer day.
Show Solution
The ice and liquid water are in thermal equilibrium, so that the temperature stays at the freezing temperature as long as ice remains in the liquid. (Once all of the ice melts, the water temperature will start to rise.)
Phase Change and Latent Heat
So far, we have discussed heat transfers that cause temperature change. However, in a phase transition, heat transfer does not cause any temperature change.
For an example of phase changes, consider the addition of heat to a sample of ice at [latex]-20\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] (Figure 1.15) and atmospheric pressure. The temperature of the ice rises linearly, absorbing heat at a constant rate of [latex]2090\phantom{\rule{0.2em}{0ex}}\text{J/kg}·\text{ºC}[/latex] until it reaches [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}.[/latex] Once at this temperature, the ice begins to melt and continues until it has all melted, absorbing 333 kJ/kg of heat. The temperature remains constant at [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] during this phase change. Once all the ice has melted, the temperature of the liquid water rises, absorbing heat at a new constant rate of [latex]\text{4186 J/kg}·\text{ºC}\text{.}[/latex] At [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C},[/latex] the water begins to boil. The temperature again remains constant during this phase change while the water absorbs 2256 kJ/kg of heat and turns into steam. When all the liquid has become steam, the temperature rises again, absorbing heat at a rate of [latex]2020\phantom{\rule{0.2em}{0ex}}\text{J/kg}·\text{ºC}[/latex]. If we started with steam and cooled it to make it condense into liquid water and freeze into ice, the process would exactly reverse, with the temperature again constant during each phase transition.
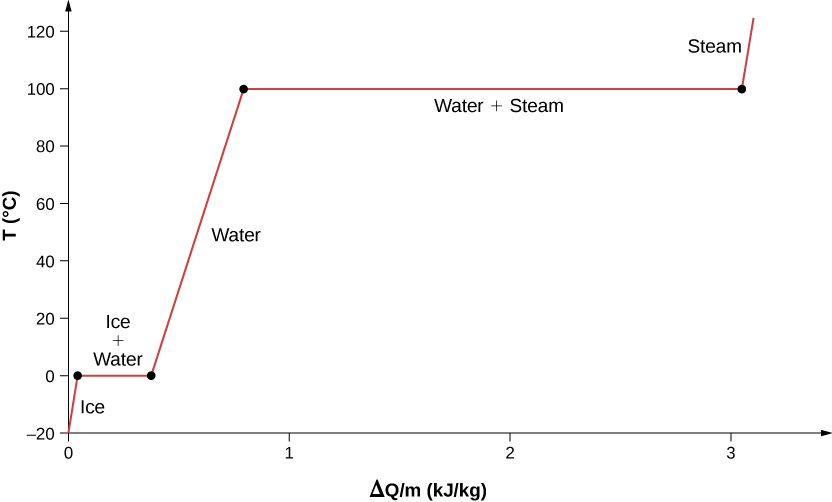
Where does the heat added during melting or boiling go, considering that the temperature does not change until the transition is complete? Energy is required to melt a solid, because the attractive forces between the molecules in the solid must be broken apart, so that in the liquid, the molecules can move around at comparable kinetic energies; thus, there is no rise in temperature. Energy is needed to vaporize a liquid for similar reasons. Conversely, work is done by attractive forces when molecules are brought together during freezing and condensation. That energy must be transferred out of the system, usually in the form of heat, to allow the molecules to stay together (Figure 1.18). Thus, condensation occurs in association with cold objects—the glass in Figure 1.16, for example.

The energy released when a liquid freezes is used by orange growers when the temperature approaches [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex]. Growers spray water on the trees so that the water freezes and heat is released to the growing oranges. This prevents the temperature inside the orange from dropping below freezing, which would damage the fruit (Figure 1.17).

The energy involved in a phase change depends on the number of bonds or force pairs and their strength. The number of bonds is proportional to the number of molecules and thus to the mass of the sample. The energy per unit mass required to change a substance from the solid phase to the liquid phase, or released when the substance changes from liquid to solid, is known as the heat of fusion. The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization. The strength of the forces depends on the type of molecules. The heat Q absorbed or released in a phase change in a sample of mass m is given by
where the latent heat of fusion [latex]{L}_{\text{f}}[/latex] and latent heat of vaporization [latex]{L}_{\text{v}}[/latex] are material constants that are determined experimentally. (Latent heats are also called latent heat coefficients and heats of transformation.) These constants are “latent,” or hidden, because in phase changes, energy enters or leaves a system without causing a temperature change in the system, so in effect, the energy is hidden.
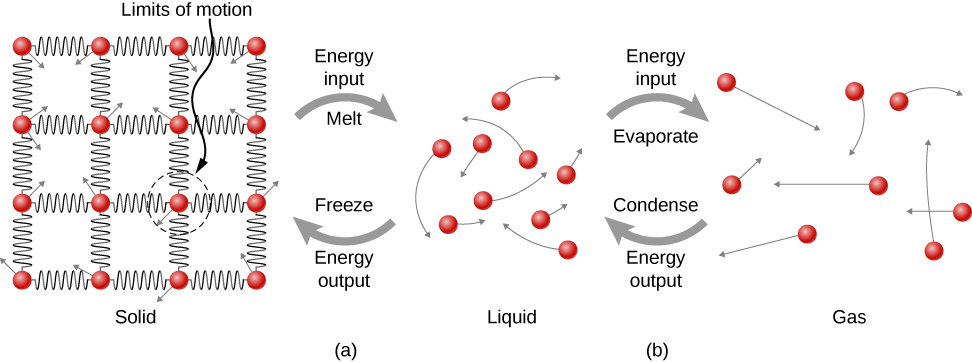
Table 1.4 lists representative values of [latex]{L}_{\text{f}}[/latex] and [latex]{L}_{\text{v}}[/latex] in kJ/kg, together with melting and boiling points. Note that in general, [latex]{L}_{\text{v}}>{L}_{\text{f}}[/latex]. The table shows that the amounts of energy involved in phase changes can easily be comparable to or greater than those involved in temperature changes, as Figure 1.15 and the accompanying discussion also showed.
| [latex]{L}_{\text{f}}[/latex] | [latex]{L}_{\text{v}}[/latex] | |||||
|---|---|---|---|---|---|---|
| Substance | Melting Point[latex]\left(\text{°C}\right)[/latex] | kJ/kg | kcal/kg | Boiling Point[latex]\left(\text{°C}\right)[/latex] | kJ/kg | kcal/kg |
| Helium[2] | [latex]-272.2\text{ }\left(0.95\text{ K}\right)[/latex] | 5.23 | 1.25 | [latex]-268.9\left(4.2\phantom{\rule{0.2em}{0ex}}\text{K}\right)[/latex] | 20.9 | 4.99 |
| Hydrogen | [latex]-259.3\left(13.9\phantom{\rule{0.2em}{0ex}}\text{K}\right)[/latex] | 58.6 | 14.0 | [latex]-252.9\left(20.2\phantom{\rule{0.2em}{0ex}}\text{K}\right)[/latex] | 452 | 108 |
| Nitrogen | [latex]-210.0\left(63.2\phantom{\rule{0.2em}{0ex}}\text{K}\right)[/latex] | 25.5 | 6.09 | [latex]-195.8\left(77.4\phantom{\rule{0.2em}{0ex}}\text{K}\right)[/latex] | 201 | 48.0 |
| Oxygen | [latex]-218.8\left(54.4\phantom{\rule{0.2em}{0ex}}\text{K}\right)[/latex] | 13.8 | 3.30 | [latex]-183.0\left(90.2\phantom{\rule{0.2em}{0ex}}\text{K}\right)[/latex] | 213 | 50.9 |
| Ethanol | –114 | 104 | 24.9 | 78.3 | 854 | 204 |
| Ammonia | –75 | 332 | 79.3 | –33.4 | 1370 | 327 |
| Mercury | –38.9 | 11.8 | 2.82 | 357 | 272 | 65.0 |
| Water | 0.00 | 334 | 79.8 | 100.0 | 2256[3] | 539[4] |
| Sulfur | 119 | 38.1 | 9.10 | 444.6 | 326 | 77.9 |
| Lead | 327 | 24.5 | 5.85 | 1750 | 871 | 208 |
| Antimony | 631 | 165 | 39.4 | 1440 | 561 | 134 |
| Aluminum | 660 | 380 | 90 | 2450 | 11400 | 2720 |
| Silver | 961 | 88.3 | 21.1 | 2193 | 2336 | 558 |
| Gold | 1063 | 64.5 | 15.4 | 2660 | 1578 | 377 |
| Copper | 1083 | 134 | 32.0 | 2595 | 5069 | 1211 |
| Uranium | 1133 | 84 | 20 | 3900 | 1900 | 454 |
| Tungsten | 3410 | 184 | 44 | 5900 | 4810 | 1150 |
Phase changes can have a strong stabilizing effect on temperatures that are not near the melting and boiling points, since evaporation and condensation occur even at temperatures below the boiling point. For example, air temperatures in humid climates rarely go above approximately [latex]38.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] because most heat transfer goes into evaporating water into the air. Similarly, temperatures in humid weather rarely fall below the dew point—the temperature where condensation occurs given the concentration of water vapor in the air—because so much heat is released when water vapor condenses.
More energy is required to evaporate water below the boiling point than at the boiling point, because the kinetic energy of water molecules at temperatures below [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] is less than that at [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex], so less energy is available from random thermal motions. For example, at body temperature, evaporation of sweat from the skin requires a heat input of 2428 kJ/kg, which is about 10% higher than the latent heat of vaporization at [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex]. This heat comes from the skin, and this evaporative cooling effect of sweating helps reduce the body temperature in hot weather. However, high humidity inhibits evaporation, so that body temperature might rise, while unevaporated sweat might be left on your brow.
Example
Calculating Final Temperature from Phase Change
Three ice cubes are used to chill a soda at [latex]20\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] with mass [latex]{m}_{\text{soda}}=0.25\phantom{\rule{0.2em}{0ex}}\text{kg}[/latex]. The ice is at [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] and each ice cube has a mass of 6.0 g. Assume that the soda is kept in a foam container so that heat loss can be ignored and that the soda has the same specific heat as water. Find the final temperature when all ice has melted.
Strategy
The ice cubes are at the melting temperature of [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}\text{.}[/latex] Heat is transferred from the soda to the ice for melting. Melting yields water at [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C},[/latex] so more heat is transferred from the soda to this water until the water plus soda system reaches thermal equilibrium.
The heat transferred to the ice is
The heat given off by the soda is
Since no heat is lost, [latex]{Q}_{\text{ice}}=\text{−}{Q}_{\text{soda}},[/latex] as in Example 1.7, so that
Solve for the unknown quantity [latex]{T}_{\text{f}}[/latex]:
Solution
Show Answer
First we identify the known quantities. The mass of ice is [latex]{m}_{\text{ice}}=3\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}6.0\phantom{\rule{0.2em}{0ex}}\text{g}=0.018\phantom{\rule{0.2em}{0ex}}\text{kg}[/latex] and the mass of soda is [latex]{m}_{\text{soda}}=0.25\phantom{\rule{0.2em}{0ex}}\text{kg}\text{.}[/latex] Then we calculate the final temperature:
Significance
This example illustrates the large energies involved during a phase change. The mass of ice is about 7% of the mass of the soda but leads to a noticeable change in the temperature of the soda. Although we assumed that the ice was at the freezing temperature, this is unrealistic for ice straight out of a freezer: The typical temperature is [latex]-6\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex]. However, this correction makes no significant change from the result we found. Can you explain why?
Like solid-liquid and and liquid-vapor transitions, direct solid-vapor transitions or sublimations involve heat. The energy transferred is given by the equation [latex]Q=m{L}_{\text{s}}[/latex], where [latex]{L}_{\text{s}}[/latex] is the heat of sublimation, analogous to [latex]{L}_{\text{f}}[/latex] and [latex]{L}_{\text{v}}[/latex]. The heat of sublimation at a given temperature is equal to the heat of fusion plus the heat of vaporization at that temperature.
We can now calculate any number of effects related to temperature and phase change. In each case, it is necessary to identify which temperature and phase changes are taking place. Keep in mind that heat transfer and work can cause both temperature and phase changes.
Problem-Solving Strategy: The Effects of Heat Transfer
- Examine the situation to determine that there is a change in the temperature or phase. Is there heat transfer into or out of the system? When it is not obvious whether a phase change occurs or not, you may wish to first solve the problem as if there were no phase changes, and examine the temperature change obtained. If it is sufficient to take you past a boiling or melting point, you should then go back and do the problem in steps—temperature change, phase change, subsequent temperature change, and so on.
- Identify and list all objects that change temperature or phase.
- Identify exactly what needs to be determined in the problem (identify the unknowns). A written list is useful.
- Make a list of what is given or what can be inferred from the problem as stated (identify the knowns). If there is a temperature change, the transferred heat depends on the specific heat of the substance (Heat Transfer, Specific Heat, and Calorimetry), and if there is a phase change, the transferred heat depends on the latent heat of the substance (Table 1.4).
- Solve the appropriate equation for the quantity to be determined (the unknown).
- Substitute the knowns along with their units into the appropriate equation and obtain numerical solutions complete with units. You may need to do this in steps if there is more than one state to the process, such as a temperature change followed by a phase change. However, in a calorimetry problem, each step corresponds to a term in the single equation [latex]{Q}_{\text{hot}}+{Q}_{\text{cold}}=0[/latex].
- Check the answer to see if it is reasonable. Does it make sense? As an example, be certain that any temperature change does not also cause a phase change that you have not taken into account.
Check Your Understanding
Why does snow often remain even when daytime temperatures are higher than the freezing temperature?
Show Solution
Snow is formed from ice crystals and thus is the solid phase of water. Because enormous heat is necessary for phase changes, it takes a certain amount of time for this heat to be transferred from the air, even if the air is above [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex].
Summary
- Most substances have three distinct phases (under ordinary conditions on Earth), and they depend on temperature and pressure.
- Two phases coexist (i.e., they are in thermal equilibrium) at a set of pressures and temperatures.
- Phase changes occur at fixed temperatures for a given substance at a given pressure, and these temperatures are called boiling, freezing (or melting), and sublimation points.
Conceptual Questions
A pressure cooker contains water and steam in equilibrium at a pressure greater than atmospheric pressure. How does this greater pressure increase cooking speed?
Show Solution
It raises the boiling point, so the water, which the food gains heat from, is at a higher temperature.
As shown below, which is the phase diagram for carbon dioxide, what is the vapor pressure of solid carbon dioxide (dry ice) at [latex]-78.5\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}?[/latex] (Note that the axes in the figure are nonlinear and the graph is not to scale.)
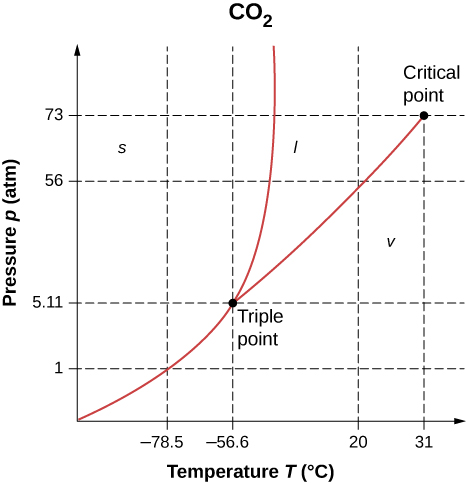
Can carbon dioxide be liquefied at room temperature ([latex]20\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex])? If so, how? If not, why not? (See the phase diagram in the preceding problem.)
Show Solution
Yes, by raising the pressure above 56 atm.
What is the distinction between gas and vapor?
Heat transfer can cause temperature and phase changes. What else can cause these changes?
Show Solution
work
How does the latent heat of fusion of water help slow the decrease of air temperatures, perhaps preventing temperatures from falling significantly below [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C,}[/latex] in the vicinity of large bodies of water?
What is the temperature of ice right after it is formed by freezing water?
Show Solution
[latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] (at or near atmospheric pressure)
If you place [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] ice into [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] water in an insulated container, what will the net result be? Will there be less ice and more liquid water, or more ice and less liquid water, or will the amounts stay the same?
What effect does condensation on a glass of ice water have on the rate at which the ice melts? Will the condensation speed up the melting process or slow it down?
Show Solution
Condensation releases heat, so it speeds up the melting.
In Miami, Florida, which has a very humid climate and numerous bodies of water nearby, it is unusual for temperatures to rise above about [latex]38\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] ([latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{F}[/latex]). In the desert climate of Phoenix, Arizona, however, temperatures rise above that almost every day in July and August. Explain how the evaporation of water helps limit high temperatures in humid climates.
In winter, it is often warmer in San Francisco than in Sacramento, 150 km inland. In summer, it is nearly always hotter in Sacramento. Explain how the bodies of water surrounding San Francisco moderate its extreme temperatures.
Show Solution
Because of water’s high specific heat, it changes temperature less than land. Also, evaporation reduces temperature rises. The air tends to stay close to equilibrium with the water, so its temperature does not change much where there’s a lot of water around, as in San Francisco but not Sacramento.
Freeze-dried foods have been dehydrated in a vacuum. During the process, the food freezes and must be heated to facilitate dehydration. Explain both how the vacuum speeds up dehydration and why the food freezes as a result.
In a physics classroom demonstration, an instructor inflates a balloon by mouth and then cools it in liquid nitrogen. When cold, the shrunken balloon has a small amount of light blue liquid in it, as well as some snow-like crystals. As it warms up, the liquid boils, and part of the crystals sublime, with some crystals lingering for a while and then producing a liquid. Identify the blue liquid and the two solids in the cold balloon. Justify your identifications using data from (Figure).
Show Solution
The liquid is oxygen, whose boiling point is above that of nitrogen but whose melting point is below the boiling point of liquid nitrogen. The crystals that sublime are carbon dioxide, which has no liquid phase at atmospheric pressure. The crystals that melt are water, whose melting point is above carbon dioxide’s sublimation point. The water came from the instructor’s breath.
Problems
How much heat transfer (in kilocalories) is required to thaw a 0.450-kg package of frozen vegetables originally at [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] if their heat of fusion is the same as that of water?
A bag containing [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] ice is much more effective in absorbing energy than one containing the same amount of [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] water. (a) How much heat transfer is necessary to raise the temperature of 0.800 kg of water from [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] to [latex]30.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex]? (b) How much heat transfer is required to first melt 0.800 kg of [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] ice and then raise its temperature? (c) Explain how your answer supports the contention that the ice is more effective.
Show Solution
a. [latex]1.00\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{5}\phantom{\rule{0.2em}{0ex}}\text{J}[/latex]; b. [latex]3.68\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{5}\phantom{\rule{0.2em}{0ex}}\text{J}[/latex]; c. The ice is much more effective in absorbing heat because it first must be melted, which requires a lot of energy, and then it gains the same amount of heat as the bag that started with water. The first [latex]2.67\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{5}\phantom{\rule{0.2em}{0ex}}\text{J}[/latex] of heat is used to melt the ice, then it absorbs the [latex]1.00\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{5}\phantom{\rule{0.2em}{0ex}}\text{J}[/latex] of heat as water.
(a) How much heat transfer is required to raise the temperature of a 0.750-kg aluminum pot containing 2.50 kg of water from [latex]30.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] to the boiling point and then boil away 0.750 kg of water? (b) How long does this take if the rate of heat transfer is 500 W?
Condensation on a glass of ice water causes the ice to melt faster than it would otherwise. If 8.00 g of vapor condense on a glass containing both water and 200 g of ice, how many grams of the ice will melt as a result? Assume no other heat transfer occurs. Use [latex]{L}_{v}[/latex] for water at [latex]37\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] as a better approximation than [latex]{L}_{v}[/latex] for water at [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex].)
Show Solution
58.1 g
On a trip, you notice that a 3.50-kg bag of ice lasts an average of one day in your cooler. What is the average power in watts entering the ice if it starts at [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] and completely melts to [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] water in exactly one day?
On a certain dry sunny day, a swimming pool’s temperature would rise by [latex]1.50\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] if not for evaporation. What fraction of the water must evaporate to carry away precisely enough energy to keep the temperature constant?
Show Solution
Let M be the mass of pool water and m be the mass of pool water that evaporates.
[latex]Mc\text{Δ}T=m{L}_{\text{V}\left(37\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}\right)}⇒\frac{m}{M}=\frac{c\text{Δ}T}{{L}_{\text{V}\left(37\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}\right)}}=\frac{\left(1.00\phantom{\rule{0.2em}{0ex}}\text{kcal/kg}·\text{°}\text{C}\right)\left(1.50\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}\right)}{580\phantom{\rule{0.2em}{0ex}}\text{kcal/kg}}=2.59\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-3}[/latex];
(Note that [latex]{L}_{\text{V}}[/latex] for water at [latex]37\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] is used here as a better approximation than [latex]{L}_{\text{V}}[/latex] for [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] water.)
(a) How much heat transfer is necessary to raise the temperature of a 0.200-kg piece of ice from [latex]-20.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] to [latex]130.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex], including the energy needed for phase changes? (b) How much time is required for each stage, assuming a constant 20.0 kJ/s rate of heat transfer? (c) Make a graph of temperature versus time for this process.
In 1986, an enormous iceberg broke away from the Ross Ice Shelf in Antarctica. It was an approximately rectangular prism 160 km long, 40.0 km wide, and 250 m thick. (a) What is the mass of this iceberg, given that the density of ice is [latex]917\phantom{\rule{0.2em}{0ex}}{\text{kg/m}}^{3}[/latex]? (b) How much heat transfer (in joules) is needed to melt it? (c) How many years would it take sunlight alone to melt ice this thick, if the ice absorbs an average of [latex]100\phantom{\rule{0.2em}{0ex}}{\text{W/m}}^{2}[/latex], 12.00 h per day?
Show Solution
a. [latex]1.47\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{15}\phantom{\rule{0.2em}{0ex}}\text{kg}[/latex]; b. [latex]4.90\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{20}\phantom{\rule{0.2em}{0ex}}\text{J}[/latex]; c. 48.5 y
How many grams of coffee must evaporate from 350 g of coffee in a 100-g glass cup to cool the coffee and the cup from [latex]95.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] to [latex]45.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex]? Assume the coffee has the same thermal properties as water and that the average heat of vaporization is 2340 kJ/kg (560 kcal/g). Neglect heat losses through processes other than evaporation, as well as the change in mass of the coffee as it cools. Do the latter two assumptions cause your answer to be higher or lower than the true answer?
(a) It is difficult to extinguish a fire on a crude oil tanker, because each liter of crude oil releases [latex]2.80\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{7}\phantom{\rule{0.2em}{0ex}}\text{J}[/latex] of energy when burned. To illustrate this difficulty, calculate the number of liters of water that must be expended to absorb the energy released by burning 1.00 L of crude oil, if the water’s temperature rises from [latex]20.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] to [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex], it boils, and the resulting steam’s temperature rises to [latex]300\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] at constant pressure. (b) Discuss additional complications caused by the fact that crude oil is less dense than water.
Show Solution
a. 9.67 L; b. Crude oil is less dense than water, so it floats on top of the water, thereby exposing it to the oxygen in the air, which it uses to burn. Also, if the water is under the oil, it is less able to absorb the heat generated by the oil.
The energy released from condensation in thunderstorms can be very large. Calculate the energy released into the atmosphere for a small storm of radius 1 km, assuming that 1.0 cm of rain is precipitated uniformly over this area.
To help prevent frost damage, 4.00 kg of water at [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] is sprayed onto a fruit tree. (a) How much heat transfer occurs as the water freezes? (b) How much would the temperature of the 200-kg tree decrease if this amount of heat transferred from the tree? Take the specific heat to be [latex]3.35\phantom{\rule{0.2em}{0ex}}\text{kJ/kg}·\text{°}\text{C}[/latex], and assume that no phase change occurs in the tree.
Show Solution
a. 319 kcal; b. [latex]2.00\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex]
A 0.250-kg aluminum bowl holding [latex]0.800\phantom{\rule{0.2em}{0ex}}\text{kg}[/latex] of soup at [latex]25.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] is placed in a freezer. What is the final temperature if 388 kJ of energy is transferred from the bowl and soup, assuming the soup’s thermal properties are the same as that of water?
A 0.0500-kg ice cube at [latex]-30.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] is placed in 0.400 kg of [latex]35.0\text{-}\text{°}\text{C}[/latex] water in a very well-insulated container. What is the final temperature?
Show Solution
First bring the ice up to [latex]0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] and melt it with heat [latex]{Q}_{1}:[/latex] 4.74 kcal. This lowers the temperature of water by [latex]\text{Δ}{T}_{2}:[/latex] [latex]23.15\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex]. Now, the heat lost by the hot water equals that gained by the cold water ([latex]{T}_{\text{f}}[/latex] is the final temperature): [latex]20.6\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex]
If you pour 0.0100 kg of [latex]20.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex] water onto a 1.20-kg block of ice (which is initially at [latex]-15.0\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex]), what is the final temperature? You may assume that the water cools so rapidly that effects of the surroundings are negligible.
Indigenous people sometimes cook in watertight baskets by placing hot rocks into water to bring it to a boil. What mass of [latex]500\text{-}\text{°}\text{C}[/latex] granite must be placed in 4.00 kg of [latex]15.0\text{-}\text{°}\text{C}[/latex] water to bring its temperature to [latex]100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}[/latex], if 0.0250 kg of water escapes as vapor from the initial sizzle? You may neglect the effects of the surroundings.
Show Solution
Let the subscripts r, e, v, and w represent rock, equilibrium, vapor, and water, respectively.
[latex]{m}_{\text{r}}{c}_{\text{r}}\left({T}_{1}-{T}_{\text{e}}\right)={m}_{\text{V}}{L}_{\text{V}}+{m}_{\text{W}}{c}_{\text{W}}\left({T}_{\text{e}}-{T}_{2}\right)[/latex];
[latex]\begin{array}{}\\ \\ \hfill {m}_{\text{r}}& =\frac{{m}_{\text{V}}{L}_{\text{V}}+{m}_{\text{W}}{c}_{\text{W}}\left({T}_{\text{e}}-{T}_{2}\right)}{{c}_{\text{r}}\left({T}_{1}-{T}_{\text{e}}\right)}\hfill \\ & =\frac{\left(0.0250\phantom{\rule{0.2em}{0ex}}\text{kg}\right)\left(2256\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{3}\phantom{\rule{0.2em}{0ex}}\text{J/kg}\right)+\left(3.975\phantom{\rule{0.2em}{0ex}}\text{kg}\right)\left(4186\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{3}\phantom{\rule{0.2em}{0ex}}\text{J/kg}·\text{°}\text{C}\right)\left(100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}-15\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}\right)}{\left(840\phantom{\rule{0.2em}{0ex}}\text{J/kg}·\text{°}\text{C}\right)\left(500\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}-100\phantom{\rule{0.2em}{0ex}}\text{°}\text{C}\right)}\hfill \\ & =4.38\phantom{\rule{0.2em}{0ex}}\text{kg}\hfill \end{array}[/latex]
What would the final temperature of the pan and water be in Table 1.4 if 0.260 kg of water were placed in the pan and 0.0100 kg of the water evaporated immediately, leaving the remainder to come to a common temperature with the pan?
Glossary
- critical point
- for a given substance, the combination of temperature and pressure above which the liquid and gas phases are indistinguishable
- critical pressure
- pressure at the critical point
- critical temperature
- temperature at the critical point
- heat of fusion
- energy per unit mass required to change a substance from the solid phase to the liquid phase, or released when the substance changes from liquid to solid
- heat of sublimation
- energy per unit mass required to change a substance from the solid phase to the vapor phase
- heat of vaporization
- energy per unit mass required to change a substance from the liquid phase to the vapor phase
- latent heat coefficient
- general term for the heats of fusion, vaporization, and sublimation
- phase diagram
- graph of pressure vs. temperature of a particular substance, showing at which pressures and temperatures the phases of the substance occur
- sublimation
- phase change from solid to gas
- vapor
- gas at a temperature below the boiling temperature
- vapor pressure
- pressure at which a gas coexists with its solid or liquid phase
Licenses and Attributions
Phase Changes. Authored by: OpenStax College. Located at: https://openstax.org/books/university-physics-volume-2/pages/1-5-phase-changes. License: CC BY: Attribution. License Terms: Download for free at https://openstax.org/books/university-physics-volume-2/pages/1-introduction

