Atomic Structure
The Exclusion Principle and the Periodic Table
Samuel J. Ling; Jeff Sanny; and William Moebs
Learning Objectives
By the end of this section, you will be able to:
- Explain the importance of Pauli’s exclusion principle to an understanding of atomic structure and molecular bonding
- Explain the structure of the periodic table in terms of the total energy, orbital angular momentum, and spin of individual electrons in an atom
- Describe the electron configuration of atoms in the periodic table
So far, we have studied only hydrogen, the simplest chemical element. We have found that an electron in the hydrogen atom can be completely specified by five quantum numbers:

To construct the ground state of a neutral multi-electron atom, imagine starting with a nucleus of charge Ze (that is, a nucleus of atomic number Z) and then adding Z electrons one by one. Assume that each electron moves in a spherically symmetrical electric field produced by the nucleus and all other electrons of the atom. The assumption is valid because the electrons are distributed randomly around the nucleus and produce an average electric field (and potential) that is spherically symmetrical. The electric potential U(r) for each electron does not follow the simple ![]() form because of interactions between electrons, but it turns out that we can still label each individual electron state by quantum numbers,
form because of interactions between electrons, but it turns out that we can still label each individual electron state by quantum numbers, ![]() . (The spin quantum number s is the same for all electrons, so it will not be used in this section.)
. (The spin quantum number s is the same for all electrons, so it will not be used in this section.)
The structure and chemical properties of atoms are explained in part by Pauli’s exclusion principle: No two electrons in an atom can have the same values for all four quantum numbers ![]() This principle is related to two properties of electrons: All electrons are identical (“when you’ve seen one electron, you’ve seen them all”) and they have half-integral spin
This principle is related to two properties of electrons: All electrons are identical (“when you’ve seen one electron, you’ve seen them all”) and they have half-integral spin ![]() Sample sets of quantum numbers for the electrons in an atom are given in (Figure). Consistent with Pauli’s exclusion principle, no two rows of the table have the exact same set of quantum numbers.
Sample sets of quantum numbers for the electrons in an atom are given in (Figure). Consistent with Pauli’s exclusion principle, no two rows of the table have the exact same set of quantum numbers.
| n | l | m | Subshell symbol | No. of electrons: subshell | No. of electrons: shell | |
|---|---|---|---|---|---|---|
| 1 | 0 | 0 | ½ | 1s | 2 | 2 |
| 1 | 0 | 0 | –½ | |||
| 2 | 0 | 0 | ½ | 2s | 2 | 8 |
| 2 | 0 | 0 | –½ | |||
| 2 | 1 | –1 | ½ | 2p | 6 | |
| 2 | 1 | –1 | –½ | |||
| 2 | 1 | 0 | ½ | |||
| 2 | 1 | 0 | –½ | |||
| 2 | 1 | 1 | ½ | |||
| 2 | 1 | 1 | –½ | |||
| 3 | 0 | 0 | ½ | 3s | 2 | 18 |
| 3 | 0 | 0 | –½ | |||
| 3 | 1 | –1 | ½ | 3p | 6 | |
| 3 | 1 | –1 | –½ | |||
| 3 | 1 | 0 | ½ | |||
| 3 | 1 | 0 | –½ | |||
| 3 | 1 | 1 | ½ | |||
| 3 | 1 | 1 | –½ | |||
| 3 | 2 | –2 | ½ | 3d | 10 | |
| 3 | 2 | –2 | –½ | |||
| 3 | 2 | –1 | ½ | |||
| 3 | 2 | –1 | –½ | |||
| 3 | 2 | 0 | ½ | |||
| 3 | 2 | 0 | –½ | |||
| 3 | 2 | 1 | ½ | |||
| 3 | 2 | 1 | –½ | |||
| 3 | 2 | 2 | ½ | |||
| 3 | 2 | 2 | –½ |
Electrons with the same principal quantum number n are said to be in the same shell, and those that have the same value of l are said to occupy the same subshell. An electron in the ![]() state of a hydrogen atom is denoted 1s, where the first digit indicates the shell
state of a hydrogen atom is denoted 1s, where the first digit indicates the shell ![]() and the letter indicates the subshell
and the letter indicates the subshell ![]() Two electrons in the
Two electrons in the ![]() state are denoted as
state are denoted as ![]() where the superscript indicates the number of electrons. An electron in the
where the superscript indicates the number of electrons. An electron in the ![]() state with
state with ![]() is denoted 2p. The combination of two electrons in the
is denoted 2p. The combination of two electrons in the ![]() and
and ![]() state, and three electrons in the
state, and three electrons in the ![]() and
and ![]() state is written as
state is written as ![]() and so on. This representation of the electron state is called the electron configuration of the atom. The electron configurations for several atoms are given in (Figure). Electrons in the outer shell of an atom are called valence electrons. Chemical bonding between atoms in a molecule are explained by the transfer and sharing of valence electrons.
and so on. This representation of the electron state is called the electron configuration of the atom. The electron configurations for several atoms are given in (Figure). Electrons in the outer shell of an atom are called valence electrons. Chemical bonding between atoms in a molecule are explained by the transfer and sharing of valence electrons.
| Element | Electron Configuration | Spin Alignment |
|---|---|---|
| H | ||
| He | ||
| Li | ||
| Be | ||
| B | ||
| C | ||
| N | ||
| O | ||
| F | ||
| Ne | ||
| Na | ||
| Mg | ||
| Al |
The maximum number of electrons in a subshell depends on the value of the angular momentum quantum number, l. For a given a value l, there are ![]() orbital angular momentum states. However, each of these states can be filled by two electrons (spin up and down,
orbital angular momentum states. However, each of these states can be filled by two electrons (spin up and down, ![]() ). Thus, the maximum number of electrons in a subshell is
). Thus, the maximum number of electrons in a subshell is
In the 2s ![]() subshell, the maximum number of electrons is 2. In the 2p (
subshell, the maximum number of electrons is 2. In the 2p (![]() ) subshell, the maximum number of electrons is 6. Therefore, the total maximum number of electrons in the
) subshell, the maximum number of electrons is 6. Therefore, the total maximum number of electrons in the ![]() shell (including both the
shell (including both the ![]() and 1 subshells) is
and 1 subshells) is ![]() or 8. In general, the maximum number of electrons in the nth shell is
or 8. In general, the maximum number of electrons in the nth shell is ![]()
Subshells and Totals for ![]() How many subshells are in the
How many subshells are in the ![]() shell? Identify each subshell and calculate the maximum number of electrons that will fill each. Show that the maximum number of electrons that fill an atom is
shell? Identify each subshell and calculate the maximum number of electrons that will fill each. Show that the maximum number of electrons that fill an atom is ![]() .
.
Strategy Subshells are determined by the value of l; thus, we first determine which values of l are allowed, and then we apply the equation “maximum number of electrons that can be in a subshell ![]() ” to find the number of electrons in each subshell.
” to find the number of electrons in each subshell.
Solution Because ![]() we know that l can be 0, 1, or 2; thus, there are three possible subshells. In standard notation, they are labeled the 3s, 3p, and 3d subshells. We have already seen that two electrons can be in an s state, and six in a p state, but let us use the equation “maximum number of electrons that can be in a subshell
we know that l can be 0, 1, or 2; thus, there are three possible subshells. In standard notation, they are labeled the 3s, 3p, and 3d subshells. We have already seen that two electrons can be in an s state, and six in a p state, but let us use the equation “maximum number of electrons that can be in a subshell ![]() ” to calculate the maximum number in each:
” to calculate the maximum number in each:
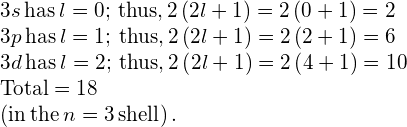
The equation “maximum number of electrons that can be in a shell ![]() ” gives the maximum number in the
” gives the maximum number in the ![]() shell to be
shell to be
Significance The total number of electrons in the three possible subshells is thus the same as the formula ![]() . In standard (spectroscopic) notation, a filled
. In standard (spectroscopic) notation, a filled ![]() shell is denoted as
shell is denoted as ![]() . Shells do not fill in a simple manner. Before the
. Shells do not fill in a simple manner. Before the ![]() shell is completely filled, for example, we begin to find electrons in the
shell is completely filled, for example, we begin to find electrons in the ![]() shell.
shell.
The structure of the periodic table ((Figure)) can be understood in terms of shells and subshells, and, ultimately, the total energy, orbital angular momentum, and spin of the electrons in the atom. A detailed discussion of the periodic table is left to a chemistry course—we sketch only its basic features here. In this discussion, we assume that the atoms are electrically neutral; that is, they have the same number of electrons and protons. (Recall that the total number of protons in an atomic nucleus is called the atomic number, Z.)
First, the periodic table is arranged into columns and rows. The table is read left to right and top to bottom in the order of increasing atomic number Z. Atoms that belong to the same column or chemical group share many of the same chemical properties. For example, the Li and Na atoms (in the first column) bond to other atoms in a similar way. The first row of the table corresponds to the 1s (![]() ) shell of an atom.
) shell of an atom.
Consider the hypothetical procedure of adding electrons, one by one, to an atom. For hydrogen (H) (upper left), the 1s shell is filled with either a spin up or down electron (![]() ). This lone electron is easily shared with other atoms, so hydrogen is chemically active. For helium (He) (upper right), the 1s shell is filled with both a spin up and a spin down (
). This lone electron is easily shared with other atoms, so hydrogen is chemically active. For helium (He) (upper right), the 1s shell is filled with both a spin up and a spin down (![]() ) electron. This “fills” the 1s shell, so a helium atom tends not to share electrons with other atoms. The helium atom is said to be chemically inactive, inert, or noble; likewise, helium gas is said to be an inert gas or noble gas.
) electron. This “fills” the 1s shell, so a helium atom tends not to share electrons with other atoms. The helium atom is said to be chemically inactive, inert, or noble; likewise, helium gas is said to be an inert gas or noble gas.
Build an atom by adding and subtracting protons, neutrons, and electrons. How does the element, charge, and mass change? Visit PhET Explorations: Build an Atom to explore the answers to these questions.
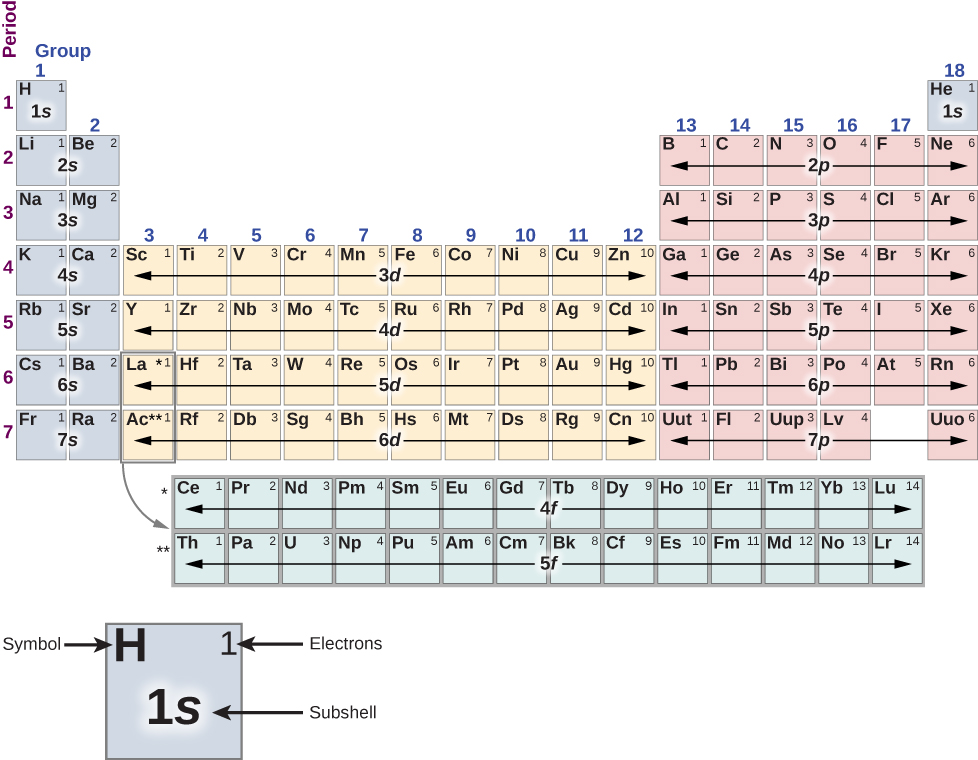
The second row corresponds to the 2s and 2p subshells. For lithium (Li) (upper left), the 1s shell is filled with a spin-up and spin-down electron (![]() ) and the 2s shell is filled with either a spin-up or -down electron (
) and the 2s shell is filled with either a spin-up or -down electron (![]() ). Its electron configuration is therefore
). Its electron configuration is therefore ![]() or [He]2s, where [He] indicates a helium core. Like hydrogen, the lone electron in the outermost shell is easily shared with other atoms. For beryllium (Be), the 2s shell is filled with a spin-up and -down electron (
or [He]2s, where [He] indicates a helium core. Like hydrogen, the lone electron in the outermost shell is easily shared with other atoms. For beryllium (Be), the 2s shell is filled with a spin-up and -down electron (![]() ), and has the electron configuration [He]
), and has the electron configuration [He]![]() .
.
Next, we look at the right side of the table. For boron (B), the 1s and 2s shells are filled and the 2p (![]() ) shell contains either a spin up or down electron (
) shell contains either a spin up or down electron (![]() ). From carbon (C) to neon (N), we the fill the 2p shell. The maximum number of electrons in the 2p shells is
). From carbon (C) to neon (N), we the fill the 2p shell. The maximum number of electrons in the 2p shells is ![]() . For neon (Ne), the 1s shell is filled with a spin-up and spin-down electron (
. For neon (Ne), the 1s shell is filled with a spin-up and spin-down electron (![]() ), and the 2p shell is filled with six electrons (
), and the 2p shell is filled with six electrons (![]() . This “fills” the 1s, 2s, and 2p subshells, so like helium, the neon atom tends not to share electrons with other atoms.
. This “fills” the 1s, 2s, and 2p subshells, so like helium, the neon atom tends not to share electrons with other atoms.
The process of electron filling repeats in the third row. However, beginning in the fourth row, the pattern is broken. The actual order of order of electron filling is given by
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s,…
Notice that the 3d, 4d, 4f, and 5d subshells (in bold) are filled out of order; this occurs because of interactions between electrons in the atom, which so far we have neglected. The transition metals are elements in the gap between the first two columns and the last six columns that contain electrons that fill the d (![]() ) subshell. As expected, these atoms are arranged in
) subshell. As expected, these atoms are arranged in ![]() columns. The structure of the periodic table can be understood in terms of the quantization of the total energy (n), orbital angular momentum (l), and spin (s). The first two columns correspond to the s
columns. The structure of the periodic table can be understood in terms of the quantization of the total energy (n), orbital angular momentum (l), and spin (s). The first two columns correspond to the s ![]() ) subshell, the next six columns correspond to the p (
) subshell, the next six columns correspond to the p (![]() ) subshell, and the gap between these columns corresponds to the d (
) subshell, and the gap between these columns corresponds to the d (![]() ) subshell.
) subshell.
The periodic table also gives information on molecular bonding. To see this, consider atoms in the left-most column (the so-called alkali metals including: Li, Na, and K). These atoms contain a single electron in the 2s subshell, which is easily donated to other atoms. In contrast, atoms in the second-to-right column (the halogens: for example, Cl, F, and Br) are relatively stingy in sharing electrons. These atoms would much rather accept an electron, because they are just one electron shy of a filled shell (“of being noble”).
Therefore, if a Na atom is placed in close proximity to a Cl atom, the Na atom freely donates its 2s electron and the Cl atom eagerly accepts it. In the process, the Na atom (originally a neutral charge) becomes positively charged and the Cl (originally a neutral charge) becomes negatively charged. Charged atoms are called ions. In this case, the ions are ![]() and
and ![]() , where the superscript indicates charge of the ion. The electric (Coulomb) attraction between these atoms forms a NaCl (salt) molecule. A chemical bond between two ions is called an ionic bond. There are many kinds of chemical bonds. For example, in an oxygen molecule
, where the superscript indicates charge of the ion. The electric (Coulomb) attraction between these atoms forms a NaCl (salt) molecule. A chemical bond between two ions is called an ionic bond. There are many kinds of chemical bonds. For example, in an oxygen molecule ![]() electrons are equally shared between the atoms. The bonding of oxygen atoms is an example of a covalent bond.
electrons are equally shared between the atoms. The bonding of oxygen atoms is an example of a covalent bond.
Summary
- Pauli’s exclusion principle states that no two electrons in an atom can have all the same quantum numbers.
- The structure of the periodic table of elements can be explained in terms of the total energy, orbital angular momentum, and spin of electrons in an atom.
- The state of an atom can be expressed by its electron configuration, which describes the shells and subshells that are filled in the atom.
Conceptual Questions
What is Pauli’s exclusion principle? Explain the importance of this principle for the understanding of atomic structure and molecular bonding.
Compare the electron configurations of the elements in the same column of the periodic table.
Elements that belong in the same column in the periodic table of elements have the same fillings of their outer shells, and therefore the same number of valence electrons. For example:
Li: ![]() (one valence electron in the
(one valence electron in the ![]() shell)
shell)
Na: ![]() (one valence electron in the
(one valence electron in the ![]() shell)
shell)
Both, Li and Na belong to first column.
Compare the electron configurations of the elements that belong in the same row of the periodic table of elements.
Problems
(a) How many electrons can be in the ![]() shell?
shell?
(b) What are its subshells, and how many electrons can be in each?
a. 32; b.
*** QuickLaTeX cannot compile formula:
\begin{array}{c}\hfill \underset{\text{_____________________}}{\begin{array}{ccccccc}\underset{_}{\ell }\hfill & & & & 2\left(2\ell +1\right)\hfill & & \\ 0\hfill & & s\hfill & & 2\left(0+1\right)\hfill & =\hfill & 2\hfill \\ 1\hfill & & p\hfill & & 2\left(2+1\right)\hfill & =\hfill & 6\hfill \\ 2\hfill & & d\hfill & & 2\left(4+1\right)\hfill & =\hfill & 10\hfill \\ 3\hfill & & f\hfill & & 2\left(6+1\right)\hfill & =\hfill & 14\hfill \end{array}}\\ \hfill 32\end{array}
*** Error message:
Missing { inserted.
leading text: ...ht)\hfill & =\hfill & 14\hfill \end{array}}
Missing } inserted.
leading text: ...ht)\hfill & =\hfill & 14\hfill \end{array}}
Missing { inserted.
leading text: ...ht)\hfill & =\hfill & 14\hfill \end{array}}
Missing } inserted.
leading text: ...ht)\hfill & =\hfill & 14\hfill \end{array}}
Missing { inserted.
leading text: ...ht)\hfill & =\hfill & 14\hfill \end{array}}
Missing } inserted.
leading text: ...ht)\hfill & =\hfill & 14\hfill \end{array}}
Missing $ inserted.
leading text: ...ht)\hfill & =\hfill & 14\hfill \end{array}}
Missing { inserted.
leading text: ...ht)\hfill & =\hfill & 14\hfill \end{array}}
Missing { inserted.
leading text: ...ht)\hfill & =\hfill & 14\hfill \end{array}}
Missing { inserted.
leading text: ...ht)\hfill & =\hfill & 14\hfill \end{array}}
(a) What is the minimum value of l for a subshell that contains 11 electrons?
(b) If this subshell is in the ![]() shell, what is the spectroscopic notation for this atom?
shell, what is the spectroscopic notation for this atom?
Unreasonable result. Which of the following spectroscopic notations are not allowed? (a) ![]() (b)
(b) ![]() (c)
(c) ![]() (d)
(d) ![]() (e)
(e) ![]() . State which rule is violated for each notation that is not allowed.
. State which rule is violated for each notation that is not allowed.
a. and e. are allowed; the others are not allowed.
b. ![]() not allowed for
not allowed for ![]()
c. Cannot have three electrons in s subshell because ![]()
d. Cannot have seven electrons in p subshell (max of 6) ![]()
Write the electron configuration for potassium.
Write the electron configuration for iron.
![]()
The valence electron of potassium is excited to a 5d state. (a) What is the magnitude of the electron’s orbital angular momentum? (b) How many states are possible along a chosen direction?
(a) If one subshell of an atom has nine electrons in it, what is the minimum value of l? (b) What is the spectroscopic notation for this atom, if this subshell is part of the ![]() shell?
shell?
a. The minimum value of ![]() is
is ![]() to have nine electrons in it.
to have nine electrons in it.
b. ![]()
Write the electron configuration for magnesium.
Write the electron configuration for carbon.
![]()
The magnitudes of the resultant spins of the electrons of the elements B through Ne when in the ground state are: ![]()
![]() ,
, ![]()
![]() ,
, ![]() and 0, respectively. Argue that these spins are consistent with Hund’s rule.
and 0, respectively. Argue that these spins are consistent with Hund’s rule.
Glossary
- chemical group
- group of elements in the same column of the periodic table that possess similar chemical properties
- covalent bond
- chemical bond formed by the sharing of electrons between two atoms
- electron configuration
- representation of the state of electrons in an atom, such as
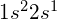 for lithium
for lithium
- ionic bond
- chemical bond formed by the electric attraction between two oppositely charged ions
- Pauli’s exclusion principle
- no two electrons in an atom can have the same values for all four quantum numbers
- transition metal
- element that is located in the gap between the first two columns and the last six columns of the table of elements that contains electrons that fill the d subshell
- valence electron
- electron in the outer shell of an atom that participates in chemical bonding

