Condensed Matter Physics
Bonding in Crystalline Solids
Samuel J. Ling; Jeff Sanny; and William Moebs
Learning Objectives
By the end of this section, you will be able to:
- Describe the packing structures of common solids
- Explain the difference between bonding in a solid and in a molecule
- Determine the equilibrium separation distance given crystal properties
- Determine the dissociation energy of a salt given crystal properties
Beginning in this section, we study crystalline solids, which consist of atoms arranged in an extended regular pattern called a lattice. Solids that do not or are unable to form crystals are classified as amorphous solids. Although amorphous solids (like glass) have a variety of interesting technological applications, the focus of this chapter will be on crystalline solids.
Atoms arrange themselves in a lattice to form a crystal because of a net attractive force between their constituent electrons and atomic nuclei. The crystals formed by the bonding of atoms belong to one of three categories, classified by their bonding: ionic, covalent, and metallic. Molecules can also bond together to form crystals; these bonds, not discussed here, are classified as molecular. Early in the twentieth century, the atomic model of a solid was speculative. We now have direct evidence of atoms in solids ((Figure)).
Ionic Bonding in Solids
Many solids form by ionic bonding. A prototypical example is the sodium chloride crystal, as we discussed earlier. Electrons transfer from sodium atoms to adjacent chlorine atoms, since the valence electrons in sodium are loosely bound and chlorine has a large electron affinity. The positively charged sodium ions and negatively charged chlorine (chloride) ions organize into an extended regular array of atoms ((Figure)).
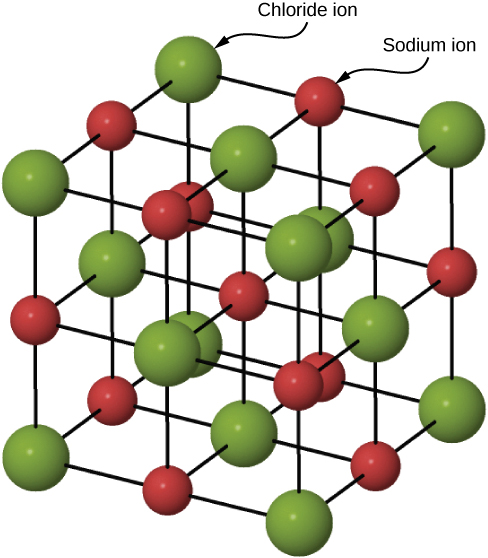
The charge distributions of the sodium and chloride ions are spherically symmetric, and the chloride ion is about two times the diameter of the sodium ion. The lowest energy arrangement of these ions is called the face-centered cubic (FCC) structure. In this structure, each ion is closest to six ions of the other species. The unit cell is a cube—an atom occupies the center and corners of each “face” of the cube. The attractive potential energy of the ![]() ion due to the fields of these six
ion due to the fields of these six ![]() ions is written
ions is written
where the minus sign designates an attractive potential (and we identify ![]() ). At a distance
). At a distance ![]() are its next-nearest neighbors: twelve
are its next-nearest neighbors: twelve ![]() ions of the same charge. The total repulsive potential energy associated with these ions is
ions of the same charge. The total repulsive potential energy associated with these ions is
Next closest are eight ![]() ions a distance
ions a distance ![]() from the
from the ![]() ion. The potential energy of the
ion. The potential energy of the ![]() ion in the field of these eight ions is
ion in the field of these eight ions is
Continuing in the same manner with alternate sets of ![]() and
and ![]() ions, we find that the net attractive potential energy
ions, we find that the net attractive potential energy ![]() of the single
of the single ![]() ion can be written as
ion can be written as
where ![]() is the Madelung constant, introduced earlier. From this analysis, we can see that this constant is the infinite converging sum
is the Madelung constant, introduced earlier. From this analysis, we can see that this constant is the infinite converging sum
Distant ions make a significant contribution to this sum, so it converges slowly, and many terms must be used to calculate ![]() accurately. For all FCC ionic solids,
accurately. For all FCC ionic solids, ![]() is approximately 1.75.
is approximately 1.75.
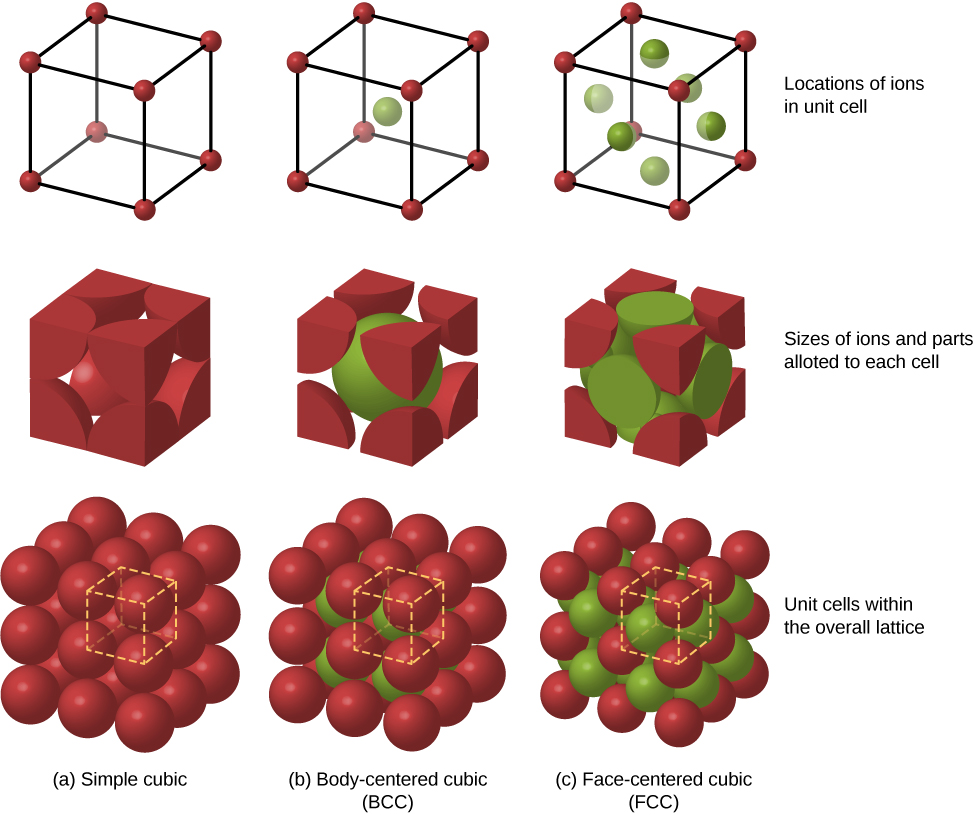
Other possible packing arrangements of atoms in solids include simple cubic and body-centered cubic (BCC). These three different packing structures of solids are compared in (Figure). The first row represents the location, but not the size, of the ions; the second row indicates the unit cells of each structure or lattice; and the third row represents the location and size of the ions. The BCC structure has eight nearest neighbors, with a Madelung constant of about 1.76—only slightly different from that for the FCC structure. Determining the Madelung constant for specific solids is difficult work and the subject of current research.
The energy of the sodium ions is not entirely due to attractive forces between oppositely charged ions. If the ions are bought too close together, the wave functions of core electrons of the ions overlap, and the electrons repel due to the exclusion principle. The total potential energy of the ![]() ion is therefore the sum of the attractive Coulomb potential
ion is therefore the sum of the attractive Coulomb potential ![]() and the repulsive potential associated with the exclusion principle
and the repulsive potential associated with the exclusion principle ![]() Calculating this repulsive potential requires powerful computers. Fortunately, however, this energy can be described accurately by a simple formula that contains adjustable parameters:
Calculating this repulsive potential requires powerful computers. Fortunately, however, this energy can be described accurately by a simple formula that contains adjustable parameters:
where the parameters A and n are chosen to give predictions consistent with experimental data. For the problem at the end of this chapter, the parameter n is referred to as the repulsion constant. The total potential energy of the ![]() ion is therefore
ion is therefore
At equilibrium, there is no net force on the ion, so the distance between neighboring ![]() and
and ![]() ions must be the value
ions must be the value ![]() for which U is a minimum. Setting
for which U is a minimum. Setting ![]() , we have
, we have
Thus,
Inserting this expression into the expression for the total potential energy, we have
Notice that the total potential energy now has only one adjustable parameter, n. The parameter A has been replaced by a function involving ![]() , the equilibrium separation distance, which can be measured by a diffraction experiment (you learned about diffraction in a previous chapter). The total potential energy is plotted in (Figure) for
, the equilibrium separation distance, which can be measured by a diffraction experiment (you learned about diffraction in a previous chapter). The total potential energy is plotted in (Figure) for ![]() , the approximate value of n for NaCl.
, the approximate value of n for NaCl.
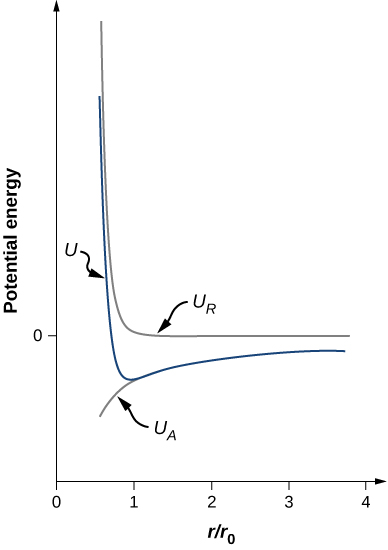
As long as ![]() , the curve for U has the same general shape: U approaches infinity as
, the curve for U has the same general shape: U approaches infinity as ![]() and U approaches zero as
and U approaches zero as ![]() . The minimum value of the potential energy is given by
. The minimum value of the potential energy is given by
The energy per ion pair needed to separate the crystal into ions is therefore
This is the dissociation energy of the solid. The dissociation energy can also be used to describe the total energy needed to break a mole of a solid into its constituent ions, often expressed in kJ/mole. The dissociation energy can be determined experimentally using the latent heat of vaporization. Sample values are given in the following table.
Thus, we can determine the Madelung constant from the crystal structure and n from the lattice energy. For NaCl, we have ![]() ,
, ![]() , and
, and ![]() This dissociation energy is relatively large. The most energetic photon from the visible spectrum, for example, has an energy of approximately
This dissociation energy is relatively large. The most energetic photon from the visible spectrum, for example, has an energy of approximately
Because the ions in crystals are so tightly bound, ionic crystals have the following general characteristics:
- They are fairly hard and stable.
- They vaporize at relatively high temperatures (1000 to 2000 K).
- They are transparent to visible radiation, because photons in the visible portion of the spectrum are not energetic enough to excite an electron from its ground state to an excited state.
- They are poor electrical conductors, because they contain effectively no free electrons.
- They are usually soluble in water, because the water molecule has a large dipole moment whose electric field is strong enough to break the electrostatic bonds between the ions.
The Dissociation Energy of Salt Determine the dissociation energy of sodium chloride (NaCl) in kJ/mol. (Hint: The repulsion constant n of NaCl is approximately 8.)
Strategy A sodium chloride crystal has an equilibrium separation of 0.282 nm. (Compare this value with 0.236 nm for a free diatomic unit of NaCl.) The dissociation energy depends on the separation distance, repulsion constant, and Madelung constant for an FCC structure. The separation distance depends in turn on the molar mass and measured density. We can determine the separation distance, and then use this value to determine the dissociation energy for one mole of the solid.
Solution The atomic masses of Na and Cl are 23.0 u and 58.4 u, so the molar mass of NaCl is 58.4 g/mol. The density of NaCl is ![]() . The relationship between these quantities is
. The relationship between these quantities is
where M is the mass of one mole of salt, ![]() is Avogadro’s number, and
is Avogadro’s number, and ![]() is the equilibrium separation distance. The factor 2 is needed since both the sodium and chloride ions represent a cubic volume
is the equilibrium separation distance. The factor 2 is needed since both the sodium and chloride ions represent a cubic volume ![]() . Solving for the distance, we get
. Solving for the distance, we get
or
The potential energy of one ion pair ![]() is
is
where ![]() is the Madelung constant,
is the Madelung constant, ![]() is the equilibrium separation distance, and n is the repulsion constant. NaCl is FCC, so the Madelung constant is
is the equilibrium separation distance, and n is the repulsion constant. NaCl is FCC, so the Madelung constant is ![]() Substituting these values, we get
Substituting these values, we get
The dissociation energy of one mole of sodium chloride is therefore
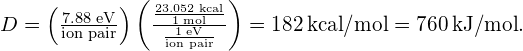
Significance This theoretical value of the dissociation energy of 766 kJ/mol is close to the accepted experimental value of 787 kJ/mol. Notice that for larger density, the equilibrium separation distance between ion pairs is smaller, as expected. This small separation distance drives up the force between ions and therefore the dissociation energy. The conversion at the end of the equation took advantage of the conversion factor ![]()
Check Your Understanding If the dissociation energy were larger, would that make it easier or more difficult to break the solid apart?
more difficult
Covalent Bonding in Solids
Crystals can also be formed by covalent bonding. For example, covalent bonds are responsible for holding carbon atoms together in diamond crystals. The electron configuration of the carbon atom is ![]() —a He core plus four valence electrons. This electron configuration is four electrons short of a full shell, so by sharing these four electrons with other carbon atoms in a covalent bond, the shells of all carbon atoms are filled. Diamond has a more complicated structure than most ionic crystals ((Figure)). Each carbon atom is the center of a regular tetrahedron, and the angle between the bonds is
—a He core plus four valence electrons. This electron configuration is four electrons short of a full shell, so by sharing these four electrons with other carbon atoms in a covalent bond, the shells of all carbon atoms are filled. Diamond has a more complicated structure than most ionic crystals ((Figure)). Each carbon atom is the center of a regular tetrahedron, and the angle between the bonds is ![]() This angle is a direct consequence of the directionality of the p orbitals of carbon atoms.
This angle is a direct consequence of the directionality of the p orbitals of carbon atoms.
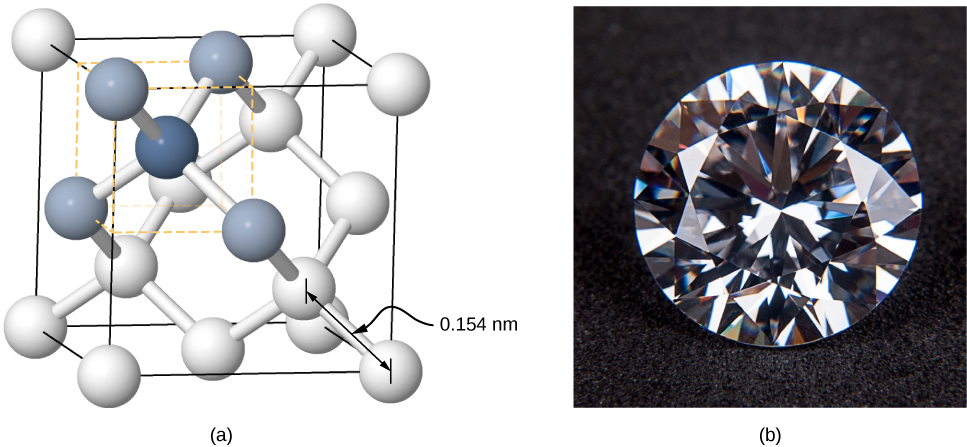
Covalently bonded crystals are not as uniform as ionic crystals but are reasonably hard, difficult to melt, and are insoluble in water. For example, diamond has an extremely high melting temperature (4000 K) and is transparent to visible light. In comparison, covalently bonded tin (also known as alpha-tin, which is nonmetallic) is relatively soft, melts at 600 K, and reflects visible light. Two other important examples of covalently bonded crystals are silicon and germanium. Both of these solids are used extensively in the manufacture of diodes, transistors, and integrated circuits. We will return to these materials later in our discussion of semiconductors.
Metallic Bonding in Solids
As the name implies, metallic bonding is responsible for the formation of metallic crystals. The valence electrons are essentially free of the atoms and are able to move relatively easily throughout the metallic crystal. Bonding is due to the attractive forces between the positive ions and the conduction electrons. Metallic bonds are weaker than ionic or covalent bonds, with dissociation energies in the range ![]() .
.
Summary
- Packing structures of common ionic salts include FCC and BCC.
- The density of a crystal is inversely related to the equilibrium constant.
- The dissociation energy of a salt is large when the equilibrium separation distance is small.
- The densities and equilibrium radii for common salts (FCC) are nearly the same.
Conceptual Questions
Why is the equilibrium separation distance between ![]() different for a diatomic molecule than for solid KCl?
different for a diatomic molecule than for solid KCl?
Each ion is in the field of multiple ions of the other opposite charge.
Describe the difference between a face-centered cubic structure (FCC) and a body-centered cubic structure (BCC).
In sodium chloride, how many ![]() atoms are “nearest neighbors” of
atoms are “nearest neighbors” of ![]() ? How many
? How many ![]() atoms are “nearest neighbors” of
atoms are “nearest neighbors” of ![]() ?
?
6, 6
In cesium iodide, how many ![]() atoms are “nearest neighbors” of
atoms are “nearest neighbors” of ![]() ? How many
? How many ![]() atoms are “nearest neighbors” of
atoms are “nearest neighbors” of ![]() ?
?
The NaCl crystal structure is FCC. The equilibrium spacing is ![]() . If each ion occupies a cubic volume of
. If each ion occupies a cubic volume of ![]() , estimate the distance between “nearest neighbor”
, estimate the distance between “nearest neighbor” ![]() ions (center-to-center)?
ions (center-to-center)?
0.399 nm
Problems
The CsI crystal structure is BCC. The equilibrium spacing is approximately ![]() . If
. If ![]() ion occupies a cubic volume of
ion occupies a cubic volume of ![]() , what is the distance of this ion to its “nearest neighbor”
, what is the distance of this ion to its “nearest neighbor” ![]() ion?
ion?
0.65 nm
The potential energy of a crystal is ![]() /ion pair. Find the dissociation energy for four moles of the crystal.
/ion pair. Find the dissociation energy for four moles of the crystal.
The measured density of a NaF crystal is ![]() . What is the equilibrium separate distance of
. What is the equilibrium separate distance of ![]() and
and ![]() ions?
ions?
![]()
What value of the repulsion constant, n, gives the measured dissociation energy of 221 kcal/mole for NaF?
Determine the dissociation energy of 12 moles of sodium chloride (NaCl). (Hint: the repulsion constant n is approximately 8.)
2196 kcal
The measured density of a KCl crystal is ![]() What is the equilibrium separation distance of
What is the equilibrium separation distance of ![]() and
and ![]() ions?
ions?
What value of the repulsion constant, n, gives the measured dissociation energy of 171 kcal/mol for KCl?
11.5
The measured density of a CsCl crystal is ![]() . What is the equilibrium separate distance of
. What is the equilibrium separate distance of ![]() and
and ![]() ions?
ions?
Glossary
- body-centered cubic (BCC)
- crystal structure in which an ion is surrounded by eight nearest neighbors located at the corners of a unit cell
- face-centered cubic (FCC)
- crystal structure in which an ion is surrounded by six nearest neighbors located at the faces at the faces of a unit cell
- lattice
- regular array or arrangement of atoms into a crystal structure
- repulsion constant
- experimental parameter associated with a repulsive force between ions brought so close together that the exclusion principle is important
- simple cubic
- basic crystal structure in which each ion is located at the nodes of a three-dimensional grid

