Chapter 4 The Second Law of Thermodynamics
4.4 Statements of the Second Law of Thermodynamics
OpenStax and Paula Herrera-Siklody
Learning Objectives
By the end of this section, you will be able to:
- Contrast the second law of thermodynamics statements according to Kelvin and Clausius formulations
- Interpret the second of thermodynamics via irreversibility
Earlier in this chapter, we introduced the Clausius statement of the second law of thermodynamics, which is based on the irreversibility of spontaneous heat flow. As we remarked then, the second law of thermodynamics can be stated in several different ways, and all of them can be shown to imply the others. In terms of heat engines, the second law of thermodynamics may be stated as follows:
This is known as the Kelvin statement of the second law of thermodynamics. This statement describes an unattainable “perfect engine,” as represented schematically in Figure 4.8(a). Note that “without any other effect” is a very strong restriction. For example, an engine can absorb heat and turn it all into work, but not if it completes a cycle. Without completing a cycle, the substance in the engine is not in its original state and therefore an “other effect” has occurred. Another example is a chamber of gas that can absorb heat from a heat reservoir and do work isothermally against a piston as it expands. However, if the gas were returned to its initial state (that is, made to complete a cycle), it would have to be compressed and heat would have to be extracted from it.
The Kelvin statement is a manifestation of a well-known engineering problem. Despite advancing technology, we are not able to build a heat engine that is [latex]100\%[/latex] efficient. The first law does not exclude the possibility of constructing a perfect engine, but the second law forbids it.
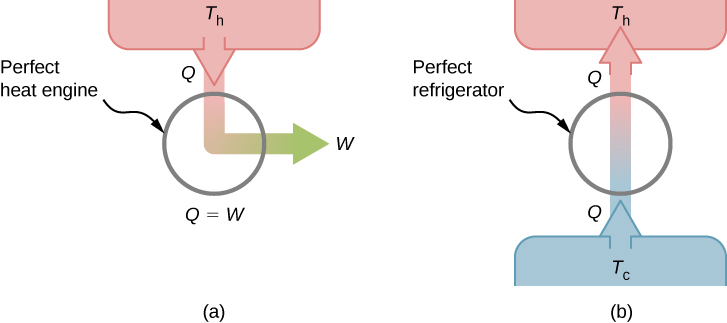
We can show that the Kelvin statement is equivalent to the Clausius statement if we view the two objects in the Clausius statement as a cold reservoir and a hot reservoir. Thus, the Clausius statement becomes: It is impossible to construct a refrigerator that transfers heat from a cold reservoir to a hot reservoir without aid from an external source. The Clausius statement is related to the everyday observation that heat never flows spontaneously from a cold object to a hot object. Heat transfer in the direction of increasing temperature always requires some energy input. A “perfect refrigerator,” shown in Figure 4.8(b), which works without such external aid, is impossible to construct.
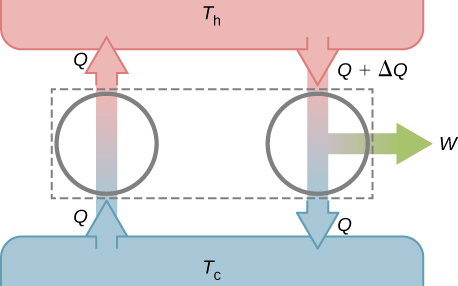
To prove the equivalence of the Kelvin and Clausius statements, we show that if one statement is false, it necessarily follows that the other statement is also false. Let us first assume that the Clausius statement is false, so that the perfect refrigerator of Figure 4.8(b) does exist. The refrigerator removes heat Q from a cold reservoir at a temperature [latex]T_\text{c}[/latex] and transfers all of it to a hot reservoir at a temperature [latex]T_\text{h}[/latex]. Now consider a real heat engine working in the same temperature range. It extracts heat [latex]Q + \Delta Q[/latex] from the hot reservoir, does work W, and discards heat Q to the cold reservoir. From the first law, these quantities are related by [latex]W = (Q + \Delta Q) - Q = \Delta Q[/latex].
Suppose these two devices are combined as shown in Figure 4.9. The net heat removed from the hot reservoir is [latex]\Delta Q[/latex], no net heat transfer occurs to or from the cold reservoir, and work W is done on some external body. Since [latex]W = \Delta Q[/latex], the combination of a perfect refrigerator and a real heat engine is itself a perfect heat engine, thereby contradicting the Kelvin statement. Thus, if the Clausius statement is false, the Kelvin statement must also be false.
Using the second law of thermodynamics, we now prove two important properties of heat engines operating between two heat reservoirs. The first property is that any reversible engine operating between two reservoirs has a greater efficiency than any irreversible engine operating between the same two reservoirs.
The second property to be demonstrated is that all reversible engines operating between the same two reservoirs have the same efficiency. To show this, we start with the two engines D and E of Figure 4.10(a), which are operating between two common heat reservoirs at temperatures [latex]T_\text{h}[/latex] and [latex]T_\text{c}[/latex]. First, we assume that D is a reversible engine and that E is a hypothetical irreversible engine that has a higher efficiency than D. If both engines perform the same amount of work W per cycle, it follows from Equation 4.2 that [latex]Q_\text{h} > Q^\prime_\text{h}[/latex]. It then follows from the first law that [latex]Q_\text{c} > Q^\prime_\text{c}[/latex].
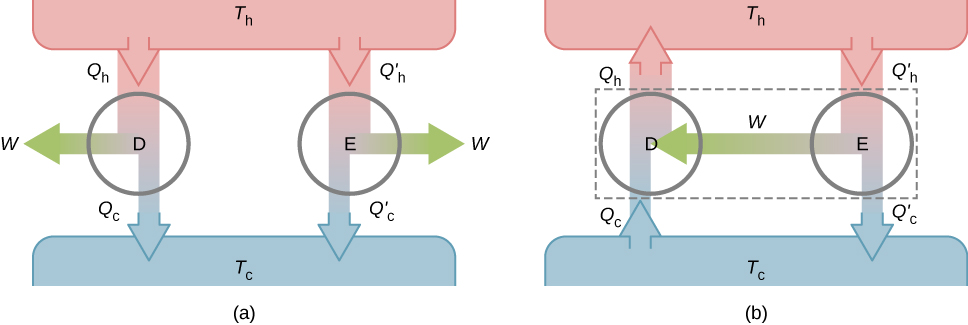
Suppose the cycle of D is reversed so that it operates as a refrigerator, and the two engines are coupled such that the work output of E is used to drive D, as shown in Figure 4.10(b). Since [latex]Q_\text{h} > Q^\prime_\text{h}[/latex] and [latex]Q_\text{c} > Q^\prime_\text{c}[/latex], the net result of each cycle is equivalent to a spontaneous transfer of heat from the cold reservoir to the hot reservoir, a process the second law does not allow. The original assumption must therefore be wrong, and it is impossible to construct an irreversible engine such that E is more efficient than the reversible engine D.
Now it is quite easy to demonstrate that the efficiencies of all reversible engines operating between the same reservoirs are equal. Suppose that D and E are both reversible engines. If they are coupled as shown in Figure 4.10(b), the efficiency of E cannot be greater than the efficiency of D, or the second law would be violated. If both engines are then reversed, the same reasoning implies that the efficiency of D cannot be greater than the efficiency of E. Combining these results leads to the conclusion that all reversible engines working between the same two reservoirs have the same efficiency.
Check Your Understanding 4.1
What is the efficiency of a perfect heat engine? What is the coefficient of performance of a perfect refrigerator?
Check Your Understanding 4.2
Show that [latex]Q_\text{h} - Q^\prime_\text{h} = Q_\text{c} - Q^\prime_\text{c}[/latex] for the hypothetical engine of Figure 4.10(b).
Media Attributions
- Figure 4.8
- Figure 4.9
- Figure 4.10

